All Photos(1)
About This Item
Empirical Formula (Hill Notation):
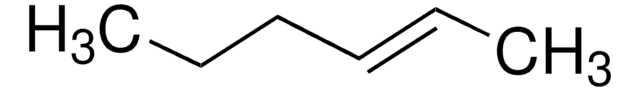
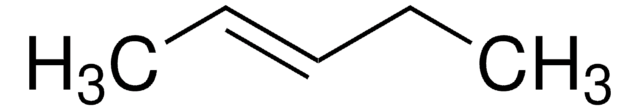
C6H12
CAS Number:
Molecular Weight:
84.16
Beilstein:
1718858
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
refractive index
n20/D 1.395
bp
66-68 °C (lit.)
density
0.681 g/mL at 20 °C (lit.)
SMILES string
CC\C=C/CC
InChI
1S/C6H12/c1-3-5-6-4-2/h5-6H,3-4H2,1-2H3/b6-5-
InChI key
ZQDPJFUHLCOCRG-WAYWQWQTSA-N
Related Categories
General description
cis-3-Hexene is a symmetrical cis-disubstituted alkene that can be prepared by the hydroboration of 3-hexyne followed by protonolysis. The gas-phase study of its molecular structure by electron diffraction combined with molecular mechanical calculations reveals the presence of the (+ac, +ac) and the (-ac, +ac) forms. cis-3-Hexene undergoes epoxidation with dimethyldioxirane to form the corresponding epoxide.
Application
cis-3-Hexene may be used in the preparation of 3-hexanol via asymmetric hydroboration with diisopinocampheylborane (Ipc2BH).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-13.0 °F - closed cup
Flash Point(C)
-25 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The molecular structures of cis-3-hexene and trans-3-hexene in the gas phase by electron diffraction and molecular mechanical calculations.
Van HD, et al.
Journal of Molecular Structure, 74(1), 123-135 (1981)
Hydroboration. 61. Diisopinocampheylborane of high optical purity. Improved preparation and asymmetric hydroboration of representative cis-disubstituted alkenes.
Brown HC, et al.
The Journal of Organic Chemistry, 47(26), 5065-5069 (1982)
Epoxidation by dimethyldioxirane. Electronic and steric effects.
Baumstark AL and Vasquez PC.
The Journal of Organic Chemistry, 53(15), 3437-3439 (1988)
Hydroboration. XI. The hydroboration of acetylenes-A convenient conversion of internal acetylenes into cis-olefins and of terminal acetylenes into aldehydes
Brown HC and Zweifel G.
Journal of the American Chemical Society, 83(18), 3834-3840 (1961)
Nadhem Aissani et al.
Journal of agricultural and food chemistry, 63(27), 6120-6125 (2015-06-18)
Research on new pesticides based on plant extracts, aimed at the development of nontoxic formulates, has recently gained increased interest. This study investigated the use of the volatilome of rucola (Eruca sativa) as a powerful natural nematicidal agent against the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service