All Photos(1)
About This Item
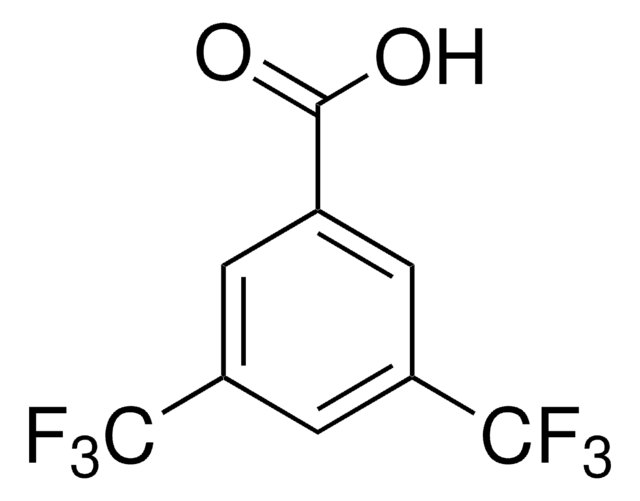
Linear Formula:
FC6H4CO2CH3
CAS Number:
Molecular Weight:
154.14
Beilstein:
1862493
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.502 (lit.)
bp
109-110 °C/35 mmHg (lit.)
density
1.21 g/mL at 25 °C (lit.)
SMILES string
COC(=O)c1ccccc1F
InChI
1S/C8H7FO2/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5H,1H3
InChI key
QAFJIJWLEBLXHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl 2-fluorobenzoate is an ortho-halogen-substituted methyl benzoate ester. It reacts with hydrazide to afford 2-fluorobenzoic hydrazide. Methyl 2-fluorobenzoate undergoes enzymatic dihydroxylation via the whole-cell fermentation in the presence of Escherichia coli JM109 (pDTG601A) to afford a diol.
Application
Methyl 2-fluorobenzoate may be used to synthesize 2-fluoro-α-methylstyrene and 2-fluorophenyldiphenylmethanol.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
200.8 °F - closed cup
Flash Point(C)
93.80 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Aromatic Fluorine Compounds. X. The 2, 3-and 2, 6-Difluoropyridines1.
Finger GC, et al.
The Journal of Organic Chemistry, 27(11), 3965-3396 (1962)
Monomers and Polymers. II. a-Methylstyrenes and the Steric Hindrance of ortho-Substituents1.
Bachman GB and Finholt RW.
Journal of the American Chemical Society, 70(2), 622-624 (1948)
The intramolecular C-F? HO hydrogen bond of 2-fluorophenyldiphenylmethanol.
Takemura H, et al.
New. J. Chem., 33(10), 2004-2006 (2009)
Vladislav Semak et al.
Organic & biomolecular chemistry, 10(22), 4407-4416 (2012-05-09)
A series of ortho-, meta-, and para- halogen-substituted methyl benzoate esters was subjected to enzymatic dihydroxylation via the whole-cell fermentation with E. coli JM109 (pDTG601A). Only ortho-substituted benzoates were metabolized. Methyl 2-fluorobenzoate yielded one diol regioselectively whereas methyl 2-chloro-, methyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service