All Photos(1)
About This Item
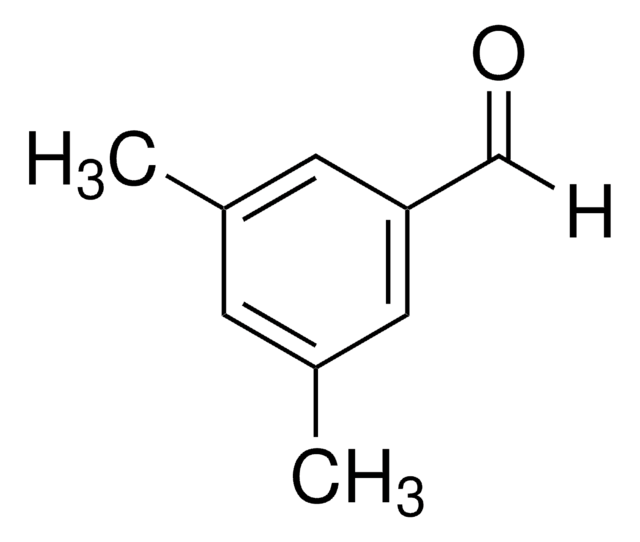
Linear Formula:
(CH3)2C6H3CHO
CAS Number:
Molecular Weight:
134.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.551 (lit.)
bp
226 °C (lit.)
density
1.012 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc(C=O)cc1C
InChI
1S/C9H10O/c1-7-3-4-9(6-10)5-8(7)2/h3-6H,1-2H3
InChI key
POQJHLBMLVTHAU-UHFFFAOYSA-N
General description
3,4-Dimethylbenzaldehyde (3,4-DMB) is a benzaldehyde derivative. It is the OH radical initiated oxidative degradation product of trimethylbenzene. The rate coefficient of the gas-phase reaction between 3,4-DMB and OH radical is 24.6±4.0×10-12cm3molecule-1s-1. The vibrational analysis of 3,4-DMB based on FT-IR spectra, FT-Raman spectra, ab initio and density functional theory (DFT) calculations have been reported. It is formed as an intermediate during the transformation of furfural into gasoline-range fuels using ZSM(Zeolite Socony Mobil)-5-based catalysts.
Application
3,4-Dimethylbenzaldehyde may be used in the synthesis of 3,4-dimethylmethcathinone (DMMC) and 3,4-dimethyl-dibenzylidene sorbitol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Urinary excretion and metabolism of the newly encountered designer drug 3,4-dimethylmethcathinone in humans.
Shima N, et al.
Forensic Toxicology, 31(1), 101-112 (2013)
Synthesis of 3,4-Dimethyl-dibenzylidene Sorbitol at Room Temperature.
Yin ZY, et al.
Huaxue Shiji, 47(3), 174-174 (2006)
Vibrational spectroscopy investigation using ab initio and density functional theory analysis on the structure of 3, 4-dimethylbenzaldehyde.
Sundaraganesan N, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 68(3), 680-687 (2007)
Rate coefficients for the gas-phase reaction of hydroxyl radicals with the dimethylbenzaldehydes.
Clifford GM and Wenger JC.
International Journal of Chemical Kinetics, 38(9), 563-569 (2006)
Catalytic fast pyrolysis of furfural over H-ZSM-5 and Zn/H-ZSM-5 catalysts.
Fanchiang WL and Lin YC.
Applied Catalysis A: General, 419, 102-110 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service