All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H11NO2
CAS Number:
Molecular Weight:
237.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
114-116 °C (lit.)
SMILES string
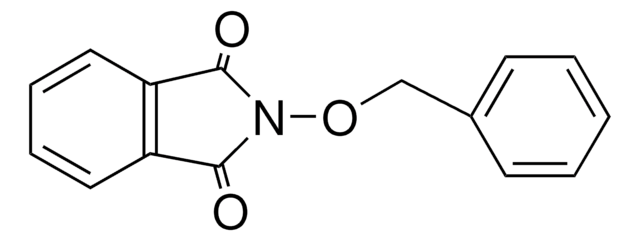
O=C1N(Cc2ccccc2)C(=O)c3ccccc13
InChI
1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)15(18)16(14)10-11-6-2-1-3-7-11/h1-9H,10H2
InChI key
WITXFYCLPDFRNM-UHFFFAOYSA-N
General description
N-Benzylphthalimide (NBPT), also known as 2-benzylisoindoline-1,3-dione, is an N-substituted phthalimide. It has been prepared by reacting phthalic anhydride with benzyl amine in glacial acetic acid. Vibrational spectra of NBPT has been recorded and assigned. NBPT is a roof-shaped molecule with a planar cyclic imide and a phenyl ring connected by a methylene group. Crystal structure of N-benzylphthalimide has parallel layers of phthalimides stack along the a axis.
Application
N-Benzylphthalimide may be used in the following syntheses:
- 2-benzyl-1,1,3,3-tetraphenylisoindoline
- tailor-made highly fluorous porphyrin derivatives
- N-benzylisoindole
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The direct conversion of phthalimides to isoindoles.
Garmaise DL and Ryan A.
Journal of Heterocyclic Chemistry, 7(2), 413-413 (1970)
Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K.
Sato HIDEO, et al.
Molecular Physics, 105(15-16), 2137-2151 (2007)
Highly fluorous porphyrins as model compounds for molecule interferometry.
Tuxen J, et al.
European Journal of Organic Chemistry, 25, 4823-4833 (2011)
Vibrational assignment of N-benzylphthalimide and 15N-benzylphthalimide.
Kolev T and Juchnovski I.
Journal of Molecular Structure, 349, 377-380 (1995)
N-benzylphthalimide.
Warzecha K-D, et al.
Acta Crystallographica Section E, Structure Reports Online, 62(6), 2367-2368 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service