342157
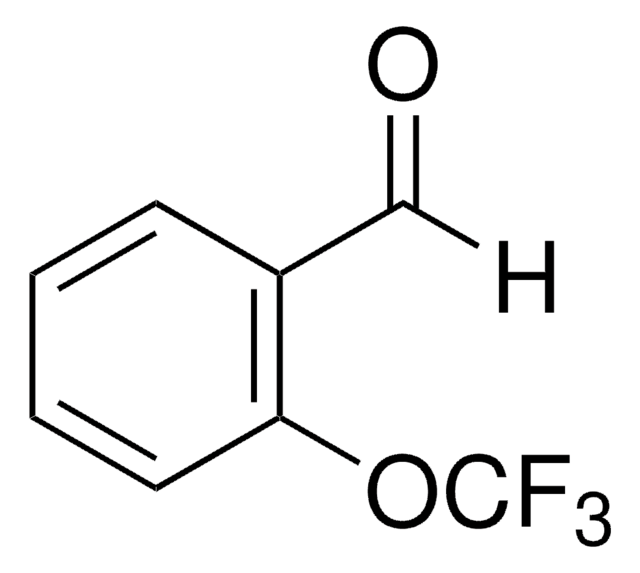
2-Hydroxy-5-(trifluoromethoxy)benzaldehyde
99%
Synonym(s):
5-(Trifluoromethoxy)salicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3OC6H3(OH)CHO
CAS Number:
Molecular Weight:
206.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
82 °C/60 mmHg (lit.)
mp
31-33 °C (lit.)
SMILES string
Oc1ccc(OC(F)(F)F)cc1C=O
InChI
1S/C8H5F3O3/c9-8(10,11)14-6-1-2-7(13)5(3-6)4-12/h1-4,13H
InChI key
WQUZBERVMUEJTD-UHFFFAOYSA-N
General description
2-Hydroxy-5-(trifluoromethoxy)benzaldehyde is formed as intermediate during the biotransformation pathways of CP-122,721 in humans.
Application
2-Hydroxy-5-(trifluoromethoxy)benzaldehyde may be used in the preparation of :
- 2-[(E)-2-hydroxy-5-(trifluoromethoxy)benzylideneamino]-4-methylphenol
- (E)-2-((3-fluorophenylimino)methyl)-4-(trifluoromethoxy) phenol
- 2-[(E)-(naphthalen-2-ylimino) methyl]-4-(trifluoromethoxy) phenol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-[(E)-(Naphthalen-2-ylimino) methyl]-4-(trifluoromethoxy) phenol.
Pekdemir M, et al.
Acta Crystallographica Section E, Structure Reports Online, 68(4), o10204-o10204 (2012)
Yelda Bingöl Alpaslan et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 3), o510-o510 (2010-01-01)
The title compound, C(14)H(9)F(4)NO(2), is a Schiff base which adopts the phenol-imine tautomeric form in the solid state. The H atom is located on the hydr-oxy O atom rather than on the N atom. This H atom is involved in
Kevin Colizza et al.
Drug metabolism and disposition: the biological fate of chemicals, 35(6), 884-897 (2007-03-16)
The metabolism, pharmacokinetics, and excretion of a potent and selective substance P receptor antagonist, CP-122,721 [(+)-(2S,3S)-3-(2-methoxy-5-trifluoromethoxybenzylamino)-2-phenylpiperidine], have been studied in six healthy male human subjects [four extensive metabolizers (EMs) and two poor metabolizers (PMs) of CYP2D6) following oral administration of
Aslı Tosyalı Karadağ et al.
Acta crystallographica. Section E, Structure reports online, 67(Pt 1), o95-o95 (2010-01-01)
The title compound, C(15)H(12)F(3)NO(3), is a Schiff base which adopts the cis-quinoid form in the solid state. The dihedral angle between the least-squares planes of the benzene rings being 3.6 (1)°. The F atoms of the -CF(3) group are disordered over
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service