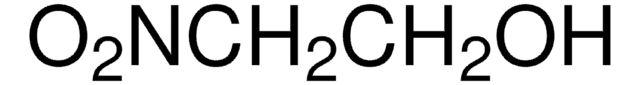308080
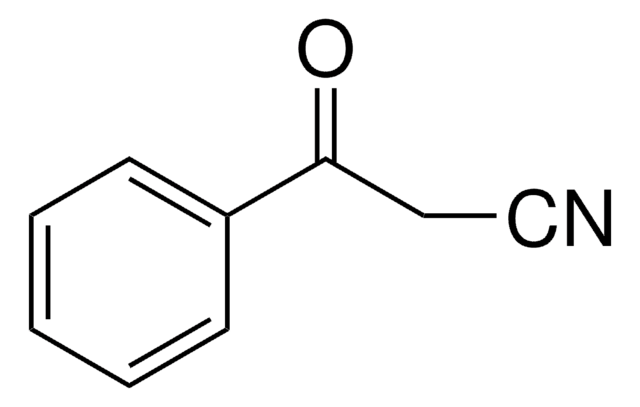
Benzoylnitromethane
98%
Synonym(s):
α-Nitroacetophenone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COCH2NO2
CAS Number:
Molecular Weight:
165.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
105-107 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)CC(=O)c1ccccc1
InChI
1S/C8H7NO3/c10-8(6-9(11)12)7-4-2-1-3-5-7/h1-5H,6H2
InChI key
JTWHVBNYYWFXSI-UHFFFAOYSA-N
Related Categories
General description
The kinetics of proton transfer from benzoylnitromethane to various bases was studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claude F. Bernasconi et al.
The Journal of organic chemistry, 62(23), 8162-8170 (2001-10-24)
The replacement of a hydrogen in nitromethane and in phenylnitromethane by the PhCO group has a strong acidifying effect, i.e., PhCOCH(2)NO(2), 5, is 5.8, 6.6, and 8.6 pK(a) units more acidic than CH(3)NO(2) in water, 50% DMSO-50% water (v/v), and
Parham Taslimi
Archiv der Pharmazie, 353(11), e2000210-e2000210 (2020-09-03)
In this study, the acetophenone derivatives 1-6 were found to be effective inhibitor molecules for α-glycosidase, human carbonic anhydrases I and II (hCA I/II), and acetylcholinesterase (AChE), with Ki values in the range of 167.98 ± 25.06 to 304.36 ± 65.45 µM for α-glycosidase, 555.76 ± 56.07
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service