All Photos(1)
About This Item
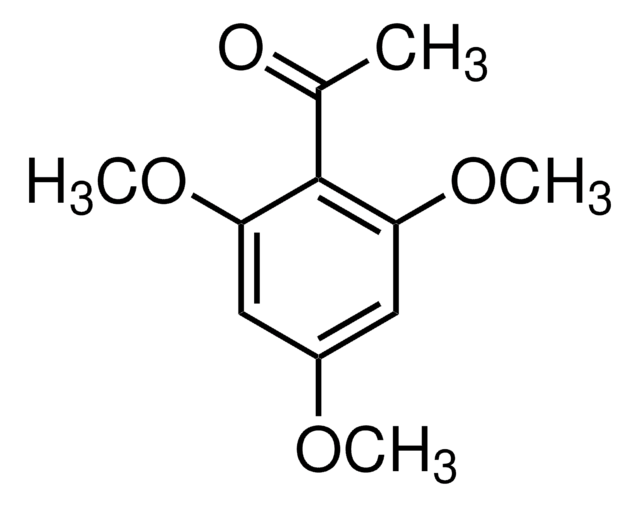
Linear Formula:
(CH3O)2C6H3COCH3
CAS Number:
Molecular Weight:
180.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
135-136 °C/2 mmHg (lit.)
mp
68-70 °C (lit.)
SMILES string
COc1cccc(OC)c1C(C)=O
InChI
1S/C10H12O3/c1-7(11)10-8(12-2)5-4-6-9(10)13-3/h4-6H,1-3H3
InChI key
XEUGKOFTNAYMMX-UHFFFAOYSA-N
General description
2,6-Dihydroxyacetophenone, its mono- and di-methyl ethers are inhibitors of hepatic mixed function oxidases. Metabolism of 2′,6′-dimethoxyacetophenone was studied.
Application
2′,6′-Dimethoxyacetophenone was used in preparation of 4-fluororesorcinol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Bobik et al.
Xenobiotica; the fate of foreign compounds in biological systems, 5(2), 65-72 (1975-02-01)
1. 2,6-Dihydroxyacetophenone, its mono- and di-methyl ethers are inhibitors of hepatic mixed function oxidases. The dimethyl ether is a competitive inhibitor of aminopyrine demethylase with the others displaying mixed kinetics. The metabolism of all three ketones has been studied. 2.
Facile preparations of 4-fluororesorcinol.
Belanger PC, et al.
Canadian Journal of Chemistry, 66(6), 1479-1482 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service