All Photos(2)
About This Item
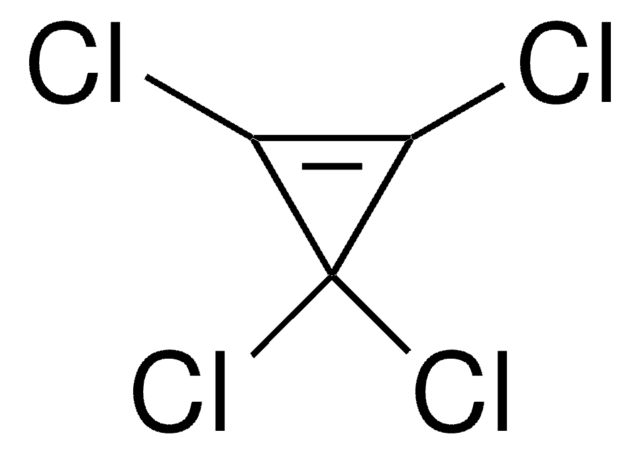
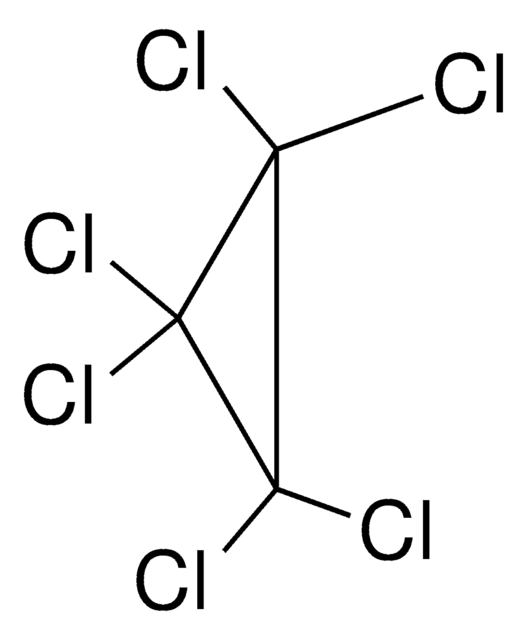
Empirical Formula (Hill Notation):
C3HCl5
CAS Number:
Molecular Weight:
214.31
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
refractive index
n20/D 1.5169 (lit.)
bp
55-56 °C/7 mmHg (lit.)
density
1.668 g/mL at 25 °C (lit.)
SMILES string
ClC1C(Cl)(Cl)C1(Cl)Cl
InChI
1S/C3HCl5/c4-1-2(5,6)3(1,7)8/h1H
InChI key
IACJMSLMMMSESC-UHFFFAOYSA-N
General description
Hydrogen-bonded complexes of pentachlorocyclopropane with the bases acetonitrile, ammonia, monomethylamine and dimethylamine have been isolated.
Application
Pentachlorocyclopropane has been used in the preparation of:
- tetrachlorocyclopropene via reaction with 18M aqueous KOH at 80-85°C
- substituted phenyltrichlorocyclopropene derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alexander B Baker et al.
The journal of physical chemistry. A, 109(37), 8280-8289 (2006-07-13)
Hydrogen-bonded complexes of pentachlorocyclopropane with the bases acetonitrile, ammonia, monomethylamine, and dimethylamine have been isolated and characterized for the first time in argon matrices at 16 K. Coordination of the proton of pentachlorocyclopropane (Pccp) to the electron donor (N) of
Synthesis of Substituted Phenyltrichlorocyclopropene Derivatives Using Friedel-Crafts Reaction.
Choi S-K and Suk W-K.
Bull. Korean Chem. Soc., 2(3), 83-85 (1981)
Tetrachlorocyclopropene and hexachlorocyclopropane from pentachlorocyclopropane.
Tobey SW and West R.
Tetrahedron Letters, 4(18), 1179-1182 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service