215333
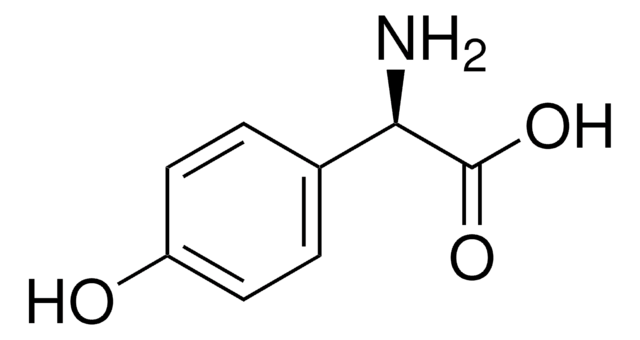
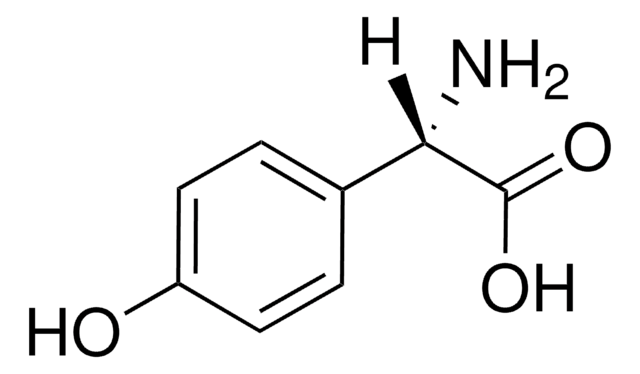
4-Hydroxy-D-phenylglycine
≥98%
Synonym(s):
(2R)-2-Amino-2-(4-hydroxyphenyl)acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H4CH(NH2)CO2H
CAS Number:
Molecular Weight:
167.16
Beilstein:
2210998
EC Number:
MDL number:
UNSPSC Code:
41116107
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
solid
optical activity
[α]23/D −158±3°, c = 1 in 1 M HCl
reaction suitability
reaction type: solution phase peptide synthesis
mp
240 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
N[C@@H](C(O)=O)c1ccc(O)cc1
InChI
1S/C8H9NO3/c9-7(8(11)12)5-1-3-6(10)4-2-5/h1-4,7,10H,9H2,(H,11,12)/t7-/m1/s1
InChI key
LJCWONGJFPCTTL-SSDOTTSWSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephan Kastner et al.
Archives of microbiology, 194(6), 557-566 (2012-02-07)
The nonproteinogenic amino acid 4-hydroxyphenylglycine (HPG) arises from the diversion of the tyrosine degradation pathway into secondary metabolism, and its biosynthesis requires a set of three enzymes. The gene cassette for HPG biosynthesis is widely spread in actinomycete bacteria, which
Mònica Prieto et al.
The Journal of organic chemistry, 74(23), 9202-9205 (2009-10-30)
Alpha-amino acid derivatives, particularly those of phenylglycine, can suffer significant racemization in Suzuki couplings. When arylpinacolboronate esters are used as coupling partners this unwanted side reaction can be suppressed by the use of Pd(OAc)(2) as Pd(0) source, in the presence
Tony Ly et al.
Journal of the American Society for Mass Spectrometry, 20(6), 1148-1158 (2009-03-17)
Photodissociation of iodo-tyrosine modified peptides yields localized radicals on the tyrosine side chain, which can be further dissociated by collisional activation. We have performed extensive experiments on model peptides, RGYALG, RGYG, and their derivatives, to elucidate the mechanisms underlying backbone
Gerald Maarman et al.
Cardiovascular drugs and therapy, 26(3), 205-216 (2012-03-13)
By increasing circulating free fatty acids and the rate of fatty acid oxidation, obesity decreases glucose oxidation and myocardial tolerance to ischemia. Partial inhibition of fatty acid oxidation may improve myocardial tolerance to ischemia/reperfusion (I/R) in obesity. We assessed the
Wendy Keung et al.
Diabetes, 62(3), 711-720 (2012-11-10)
Impaired skeletal muscle fatty acid oxidation has been suggested to contribute to insulin resistance and glucose intolerance. However, increasing muscle fatty acid oxidation may cause a reciprocal decrease in glucose oxidation, which might impair insulin sensitivity and glucose tolerance. We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service