All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H11N
CAS Number:
Molecular Weight:
157.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
57-59 °C (lit.)
SMILES string
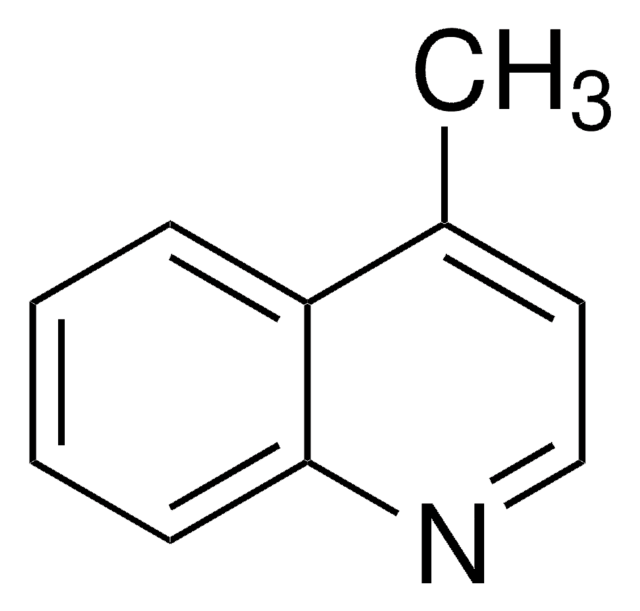
Cc1ccc2nc(C)ccc2c1
InChI
1S/C11H11N/c1-8-3-6-11-10(7-8)5-4-9(2)12-11/h3-7H,1-2H3
InChI key
JJPSZKIOGBRMHK-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Application
2,6-Dimethylquinoline was used to study the inhibition potencies (IC50 values) of structurally diverse chemicals with recombinant human CYP2B6 enzyme for in vitro research purposes.
Biochem/physiol Actions
2,6-Dimethylquinoline is the chemical constituent present in roots of Peucedantu praeruptorum. It is a potential inhibitor of cytochrome P450 1A2 activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cun Zhang et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 31(16), 1333-1335 (2006-10-26)
To isolate and identify the chemical constituents from the roots of Peucedantu praeruptorum. The constituents were isolated by column chromatography on silica gel and ODS, and identified by NMR, MS spectroscopic methods. Eight compounds, (-) sclerodin (1), palmitic acid (2)
L E Korhonen et al.
British journal of pharmacology, 150(7), 932-942 (2007-02-28)
The cytochrome P450 2B6 (CYP2B6) enzyme metabolises a number of clinically important drugs. Drug-drug interactions resulting from inhibition or induction of CYP2B6 activity may cause serious adverse effects. The aims of this study were to construct a three-dimensional structure-activity relationship
Bin Yang et al.
Environmental science and pollution research international, 23(4), 3399-3405 (2015-10-23)
The solubilities of 19 different kinds of N-heteroaromatic compounds in aqueous solutions with different concentrations of NaCl were determined at 298.15 K with a UV-vis spectrophotometry and titration method, respectively. Setschenow constants, Ks, were employed to describe the solubility behavior
Markus Brinkmann et al.
Chemical research in toxicology, 32(4), 698-707 (2019-03-22)
Hydroxylation of polyaromatic compounds through cytochromes P450 (CYPs) is known to result in potentially estrogenic transformation products. Recently, there has been an increasing awareness of the importance of alternative pathways such as aldehyde oxidases (AOX) or N-methyltransferases (NMT) in bioactivation
Laura E Korhonen et al.
Journal of medicinal chemistry, 48(11), 3808-3815 (2005-05-27)
The purpose of this study was to determine the cytochrome P450 1A2 (CYP1A2) inhibition potencies of structurally diverse compounds to create a comprehensive three-dimensional quantitative structure-activity relationship (3D-QSAR) model of CYP1A2 inhibitors and to use this model to predict the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service