All Photos(1)
About This Item
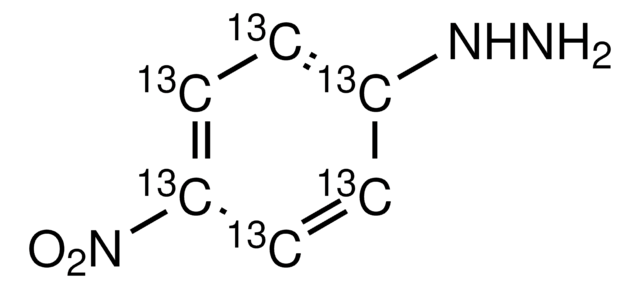
Linear Formula:
O2NC6H4NHNH2
CAS Number:
Molecular Weight:
153.14
Beilstein:
608107
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
form
solid
contains
≥10% water as stabilizer
mp
156 °C (dec.) (lit.)
SMILES string
NNc1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H7N3O2/c7-8-5-1-3-6(4-2-5)9(10)11/h1-4,8H,7H2
InChI key
KMVPXBDOWDXXEN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Expl. 1.1 - Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
1 - Explosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R V Raju et al.
Journal of AOAC International, 77(3), 748-751 (1994-05-01)
Three spectrophotometric methods were developed for the microdetermination of decamethrin in insecticidal formulations and in water. The methods are based on the hydrolysis of decamethrin with methanolic KOH to 3-phenoxybenzaldehyde; condensation of the hydrolysis product with 2,4-dinitrophenylhydrazine (2,4-DNPH), 4-nitrophenyl-hydrazine (4-NPH)
Minae Mure et al.
Journal of the American Chemical Society, 125(20), 6113-6125 (2003-06-06)
4-n-Butylamino-5-ethyl-1,2-benzoquinone (1(ox)) has been synthesized as a model compound for the LTQ (lysine tyrosyl quinone) cofactor of lysyl oxidase (LOX). At pH 7, 1(ox) has a lambda(max) at 504 nm and exists as a neutral o-quinone in contrast to a
S R Carter et al.
Journal of inorganic biochemistry, 56(2), 127-141 (1994-11-01)
An improved purification scheme for an amine oxidase from equine plasma (EPAO), a nonruminant source, is described and the protein's active-site is characterized. EPAO is dimeric and contains one Type-2 Cu(II) ion per monomer. The EPAO Cu(II) site is spectroscopically
Atsuko Satoh et al.
Biochimica et biophysica acta, 1647(1-2), 272-277 (2003-04-11)
Quinohemoprotein amine dehydrogenase (QH-AmDH) catalyzes the oxidative deamination of aliphatic and aromatic amines. The enzyme from Pseudomonas putida has an alpha beta gamma heterotrimeric structure with two heme c groups in the largest alpha subunit, and a novel quinone cofactor
I Frébort et al.
Biochemistry and molecular biology international, 36(6), 1207-1216 (1995-08-01)
Structural properties of dimeric (2 x 75 kDa) copper-containing amine oxidase (EC 1.4.3.6) from Aspergillus niger were studied. The enzyme treated with SDS was dissociated into subunits which showed different mobility on polyacrylamide gel without SDS. The separated subunits had
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service