40334
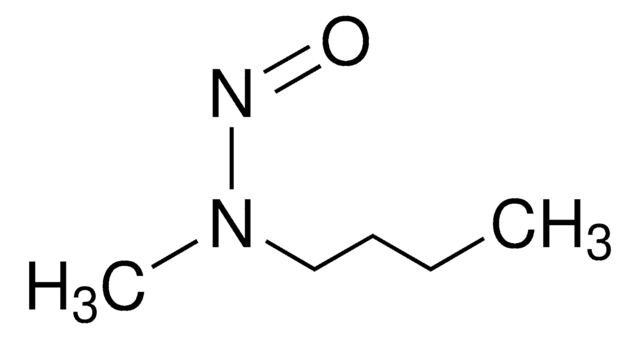
N-Nitrosodiethylamine (NDEA) solution
certified reference material, 5000 μg/mL in methanol
Synonym(s):
Diethylnitrosamine
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
CofA
current certificate can be downloaded
packaging
ampule of 1 mL
concentration
5000 μg/mL in methanol
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
cleaning products
cosmetics
environmental
food and beverages
personal care
format
single component solution
storage temp.
2-8°C
InChI
1S/C4H10N2O/c1-3-6(4-2)5-7/h3-4H2,1-2H3
InChI key
WBNQDOYYEUMPFS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Nitrosodiethylamine belongs to the group of toxic nitrosamines, known to show carcinogenic, mutagenic, and hepatotoxic properties. It is formed as a byproduct during food processing, pharmaceutical processing, and water treatment. As a result, they are generally found in water, fish, meat, drug substances, etc.
Application
- Simultaneous analysis of N-nitrosodiethanolamine, N-nitrosodiethylamine, triethanolamine, diethanolamine in cosmetic samples using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS)
- Solid phase extraction of N-nitrosodimethylamine and N-nitrosomethylethylamine from eight sartans, ranitidine, and metformin and also the fortified products of these drugs, for quantification by gas chromatography-tandem mass spectrometry (GC-MS/MS)
- Multi-residue analysis of seven restricted nitrosamines in two body creams and two shower gel samples by vortex-assisted reversed-phase dispersive liquid-liquid microextraction (VA-RP-DLLME) and liquid chromatography-mass spectrometry (LC-MS)
- Quantitative analysis of four nitrosamines in four sartan drug substances— candesartan cilexetil, olmesartan medoxomi, irbesartan, and valsartan, by gas chromatography-tandem mass spectrometry (GC-MS/MS)
- Determination of eight volatile nitrosamines in 28 popular meat product samples using ultrasonic solvent extraction combined with solid phase microextraction (SPME) and GC-MS
Other Notes
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1B - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
GC-MS method detects nitrosamines in Valsartan tablets, meeting US FDA guidelines for pharmaceutical quality control.
GC-MS method detects nitrosamines in Valsartan tablets, meeting US FDA guidelines for pharmaceutical quality control.
GC-MS method detects nitrosamines in Valsartan tablets, meeting US FDA guidelines for pharmaceutical quality control.
GC-MS method detects nitrosamines in Valsartan tablets, meeting US FDA guidelines for pharmaceutical quality control.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service