F4505
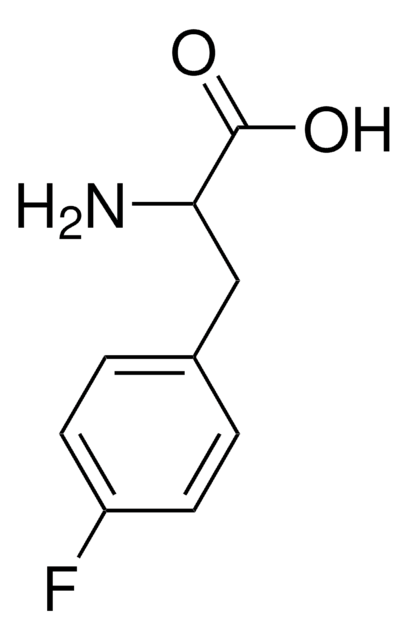
m-Fluoro-DL-tyrosine
≥98%, suitable for ligand binding assays
Synonym(s):
3-fluoro-tyrosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H3-4-(OH)CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
199.18
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
m-Fluoro-DL-tyrosine,
Assay
≥98%
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
white
mp
280 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
NC(Cc1ccc(O)c(F)c1)C(O)=O
InChI
1S/C9H10FNO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)
InChI key
VIIAUOZUUGXERI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
M-Fluorotyrosine (m-Fluoro-DL-tyrosine) may be substituted for tyrosine in the biosynthesis of proteins such as β-galactosidases (Escherichia coli), bacteriorhodopsin and intestinal microvillar enzymes, eg. aminopeptidase N, to study the effect of halogenated tryosines on the proteins properties.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Julianne L Kitevski-LeBlanc et al.
Journal of the American Chemical Society, 131(6), 2054-2055 (2009-01-29)
Solution NMR studies of protein structure and dynamics using fluorinated amino acid probes are a valuable addition to the repertoire of existing (13)C, (15)N, and (1)H experiments. Despite the numerous advantages of the (19)F nucleus in NMR, protein studies are
Julianne L Kitevski-LeBlanc et al.
Journal of biomolecular NMR, 45(3), 255-264 (2009-08-06)
Fluorine NMR is a useful tool to probe protein folding, conformation and local topology owing to the sensitivity of the chemical shift to the local electrostatic environment. As an example we make use of (19)F NMR and 3-fluorotyrosine to evaluate
Mohammad R Seyedsayamdost et al.
Nature protocols, 2(5), 1225-1235 (2007-06-05)
Expressed protein ligation (EPL) allows semisynthesis of a target protein with site-specific incorporation of probes or unnatural amino acids at its N or C termini. Here, we describe the protocol that our lab has developed for incorporating fluorotyrosines (F(n)Ys) at
C M Dupureur et al.
European journal of biochemistry, 261(1), 261-268 (1999-04-02)
The activities of restriction enzymes are important examples of Mg(II)-dependent hydrolysis of DNA. While a number of crystallographic studies of enzyme-DNA complexes have also involved metal ions, there have been no solution studies exploring the relationship between enzyme conformation and
Fabrice Rappaport et al.
Journal of the American Chemical Society, 131(12), 4425-4433 (2009-03-07)
The catalytic cycle of numerous enzymes involves the coupling between proton transfer and electron transfer. Yet, the understanding of this coordinated transfer in biological systems remains limited, likely because its characterization relies on the controlled but experimentally challenging modifications of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service