Y0000001
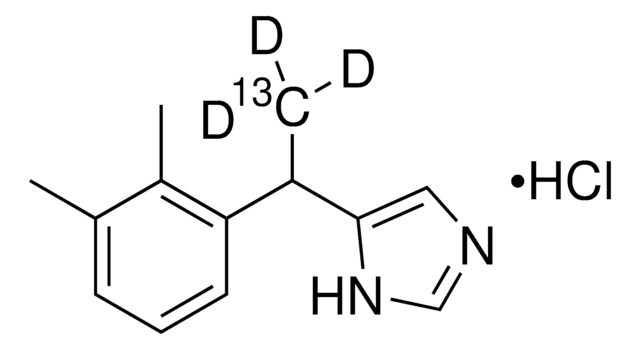
Detomidine hydrochloride
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H14N2 · HCl
CAS Number:
Molecular Weight:
222.71
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
dexmedetomidine, medetomidine
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C12H14N2.ClH/c1-9-4-3-5-11(10(9)2)6-12-7-13-8-14-12;/h3-5,7-8H,6H2,1-2H3,(H,13,14);1H
InChI key
OIWRDXKNDCJZSM-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Detomidine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Heather K Knych et al.
Veterinary anaesthesia and analgesia, 39(3), 221-229 (2012-03-13)
To describe the pharmacokinetics of detomidine and yohimbine when administered in combination. Randomized crossover design. Nine healthy adult horses aged 9 ± 4 years and weighing of 561 ± 56 kg. Three dose regimens were employed in the current study.
Assessment of the sedative effects of buprenorphine administered with 10 μg/kg detomidine in horses.
E J Love et al.
The Veterinary record, 168(14), 379-379 (2011-04-19)
The aim of this randomised, observer-blinded, crossover study was to compare the effects of six treatments, administered intravenously to six horses: saline and saline (S/S); detomidine and saline (D/S); detomidine and 5 µg/kg buprenorphine (D/B5); detomidine and 7.5 µg/kg buprenorphine
E J Love et al.
The Veterinary record, 168(15), 409-409 (2011-04-16)
The aim of this randomised, observer-blinded, crossover study was to compare the effects of four treatments, administered intravenously to six horses: saline and saline; 10 µg/kg detomidine and 7.5 µg/kg buprenorphine; 20 µg/kg detomidine and 7.5 µg/kg buprenorphine; and 20
R Buhl et al.
Veterinary journal (London, England : 1997), 196(3), 483-491 (2013-01-08)
The objective of this prospective field study was to investigate whether commonly used criteria for clinical death occurred at the same time as cardiac death, as determined by electrocardiography. Specific ECG changes during euthanasia were also studied. Twenty-nine horses were
Mari H Vainionpää et al.
Veterinary anaesthesia and analgesia, 40(3), 257-264 (2013-02-02)
To investigate plasma drug concentrations and the effect of MK-467 (L-659'066) on sedation, heart rate and gut motility in horses sedated with intravenous (IV) detomidine. Experimental randomized blinded crossover study. Six healthy horses. Detomidine (10 μg kg(-1) IV) was administered
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service