W200808
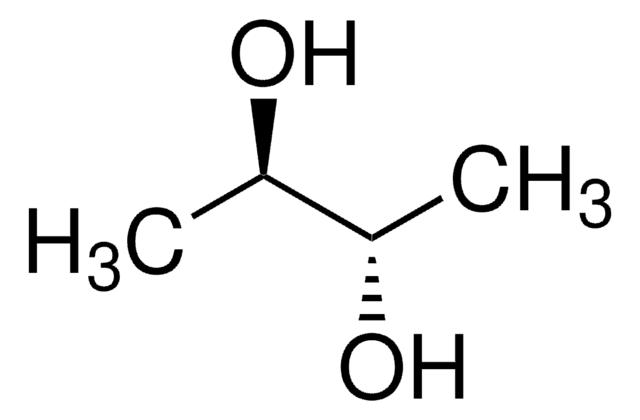
Acetoin
primarily dimer, ≥95%, FG
Synonym(s):
3-Hydroxy-2-butanone, Acetylmethylcarbinol
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 182.60
Assay
≥95%
refractive index
n20/D 1.417 (lit.)
bp
148 °C (lit.)
mp
15 °C (monomer)
90 °C (dimer) (lit.)
solubility
acetone: soluble(lit.)
water: soluble(lit.)
density
1.013 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
butter; creamy; cheesy
storage temp.
2-8°C
SMILES string
CC(O)C(C)=O
InChI
1S/C4H8O2/c1-3(5)4(2)6/h3,5H,1-2H3
InChI key
ROWKJAVDOGWPAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Role of Volatile Organic Compounds Produced by Kosakonia cowanii Cp1 during Competitive Colonization Interaction against Pectobacterium aroidearum SM2.: This study investigates the role of volatile organic compounds, including acetoin, produced by Kosakonia cowanii Cp1 in inhibiting Pectobacterium aroidearum SM2, highlighting the potential of acetoin in biocontrol applications (Mena Navarro et al., 2024).
- Chemical imitation of yeast fermentation by the drosophilid-pollinated deceptive trap-flower Aristolochia baetica (Aristolochiaceae).: The research explores how Aristolochia baetica mimics yeast fermentation, including the production of acetoin, to attract drosophilid pollinators, emphasizing acetoin′s role in plant-pollinator interactions (Rupp et al., 2024).
- Investigating the impact of various sorghum types on the key aroma compounds of Sichuan Xiaoqu Baijiu through application of the sensomics approach.: This study examines how different sorghum types influence the key aroma compounds, including acetoin, in Sichuan Xiaoqu Baijiu, demonstrating acetoin′s significance in food and beverage flavor profiles (Ma et al., 2024).
- Regulation of Tetramethylpyrazine Formation by the Phenolics-Fenton Coupled Redox Cycling System.: The research delves into the biochemical pathways regulated by acetoin in the formation of tetramethylpyrazine, providing insights into its role in flavor compound biosynthesis (Xu et al., 2024).
- Design of a synthetic enzyme cascade for the in vitro fixation of formaldehyde to acetoin.: This paper presents the development of a synthetic enzyme cascade to convert formaldehyde to acetoin, showcasing its potential in biotechnological applications for formaldehyde detoxification (Cui et al., 2024).
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service