63680
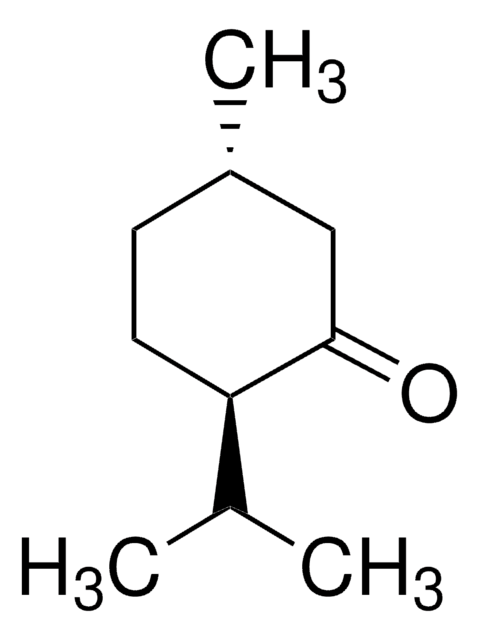
Menthone
mixture of isomers, ≥97.0% (GC)
Synonym(s):
2-Isopropyl-5-methylcyclohexanone
About This Item
Recommended Products
Quality Level
Assay
≥97.0% (GC)
bp
85-88 °C/12 mmHg (lit.)
density
0.896 g/mL at 20 °C (lit.)
SMILES string
CC(C)C1CCC(C)CC1=O
InChI
1S/C10H18O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-9H,4-6H2,1-3H3
InChI key
NFLGAXVYCFJBMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To prepare the model flavor mix in a study to investigate the release of various aroma compounds in xanthan-thickened food model systems having different viscosities.
- As standard in the quantification of volatile constituents and odour-activity value (OAV) in ′Marion′ and ′Black Diamond′.
- Modified semisolid agar antifungal susceptibility method (SAAS) to investigate the anti-fungal activities of various cyclic terpenes against Fusarium verticillioides MRC 826.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service