All Photos(2)
About This Item
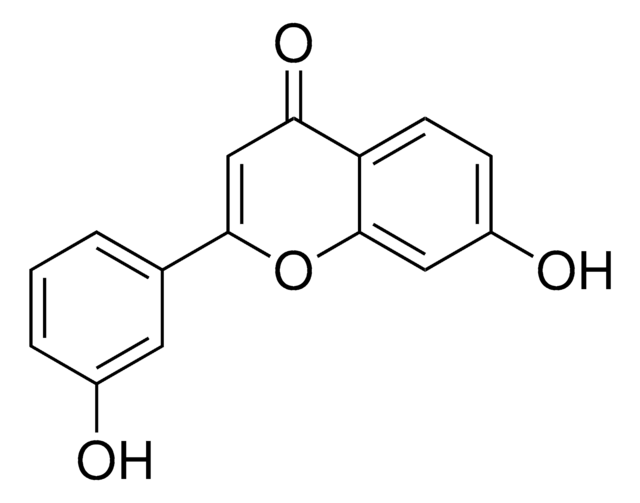
Empirical Formula (Hill Notation):
C16H12O3
CAS Number:
Molecular Weight:
252.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
163-165 °C (lit.)
functional group
ketone
phenyl
SMILES string
COc1ccc2OC(=CC(=O)c2c1)c3ccccc3
InChI
1S/C16H12O3/c1-18-12-7-8-15-13(9-12)14(17)10-16(19-15)11-5-3-2-4-6-11/h2-10H,1H3
InChI key
XZQLSABETMKIGG-UHFFFAOYSA-N
Gene Information
rat ... Gabra2(29706)
General description
6-Methoxyflavone is one of the methoxyflavone isolated form Pimelea decora. Synthesis of 6-methoxyflavone from p-dihydroxybenzene has been reported.
Application
6-Methoxyflavone may be employed in the following studies:
- As internal standard for the analysis of polyphenolic content of in-vitro-cultured chestnut shoots.
- Synthesis of biflavonoids, rac- and meso-6,6-dimethoxy-2,2-biflavanones.
- As internal standard for the separation of phenolic compounds in the apricot tissue by HPLC.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Photohydrodimerization of 6-Methoxyflavone to 6, 6′-Dimethoxy-2, 2′-Biflavanones.
Chen A-H, et al.
J. Chin. Chem. Soc., 51(6), 1389-1394 (2004)
J. L. Fernandez-Lorenzo et al.
Tree physiology, 19(7), 461-466 (2003-03-26)
The phenolic contents of eight in-vitro-cultivated chestnut clones (Castanea sativa Mill. and C. sativa x C. crenata Siebold & Zucc. hybrids) were analyzed both qualitatively and quantitatively. The aim of the work was to identify potential phenolic markers of: (i)
Pilar Errea et al.
Physiologia plantarum, 112(1), 135-141 (2001-04-25)
Graft compatibility has been studied in vitro using callus tissues of apricot (Prunus armeniaca) and different Prunus rootstocks to form scion/rootstock combinations with different degrees of graft compatibility. In these species, incompatibility is manifested by a breakdown of the trees
The constituents of Australian Pimelea species. II. The isolation of unusual flavones from P. simplex and P. decora.
Freeman PW, et al.
Australian Journal of Chemistry, 34(8), 1779-1784 (1981)
Sara Serra et al.
Foods (Basel, Switzerland), 9(10) (2020-10-18)
The rising interest in beneficial health properties of polyphenol compounds in fruit initiated this investigation about biochemical composition in peach mesocarp/exocarp. Biochemical evaluation of phenolic compounds and ascorbic acid were quantified through high-performance liquid chromatography (HPLC) in relation to three
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service