All Photos(1)
About This Item
Linear Formula:
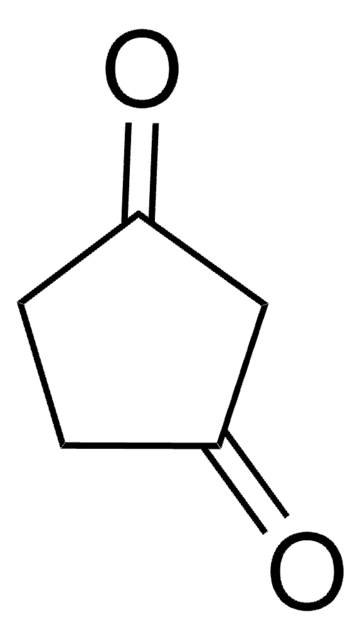
C2H5OC5H5(=O)
CAS Number:
Molecular Weight:
126.15
Beilstein:
2041482
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.492 (lit.)
bp
60-63 °C/0.3 mmHg (lit.)
density
1.062 g/mL at 25 °C (lit.)
functional group
ether
ketone
SMILES string
CCOC1=CC(=O)CC1
InChI
1S/C7H10O2/c1-2-9-7-4-3-6(8)5-7/h5H,2-4H2,1H3
InChI key
SUQNVCCJLBQVEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Ethoxy-2-cyclopentenone (3-Ethoxy-2-cyclopenten-1-one, 3-Ethoxycyclopentenone) is a cyclic β-alkoxy α,β-unsaturated ketone. Its manganese(III) acetate based tandem oxidation has been investigated. Its various physical properties such as density, refractive index and boiling point have been reported.
Application
3-Ethoxy-2-cyclopentenone (3-Ethoxy-2-cyclopenten-1-one) may be used as a starting material in the synthesis of the following:
- allylic cyclopentenyl alcohols like 2-cyclopentenol, 3-methyl-2-cyclopentenol and 3-methoxymethaoxymethyl-2-cyclopentenol
- 3-aminocyclopent-2-en-1-one
- (E)-5-(((2S*,3S*)-3-((benzyloxy)methyl)oxiran-2- yl)methylene)-3-ethoxycyclopent-2-enone, an γ,δ -epoxy-α,β-unsaturated cyclic enone
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fumihiko Yoshimura et al.
Organic & biomolecular chemistry, 10(28), 5431-5442 (2012-06-19)
We developed a new method for stereoselective construction of an all-carbon quaternary stereogenic center on a carbocyclic ring based on regio- and stereoselective S(N)2' alkylation reactions of γ,δ-epoxy-α,β-unsaturated cyclic ketones. Treatment of the ketones, which were readily prepared in enantiomerically
An efficient synthesis of 3-aminocyclopent-2-en-1-one.
Kikani BB, et al.
Synthesis, 2, 176-176 (1991)
Manganese (III) acetate based tandem oxidation of various α and β-alkoxy α, β-unsaturated ketones.
Tanyeli C, et al.
Tetrahedron, 58(50), 9983-9988 (2002)
Palladium-catalyzed rearrangements of 2-cyclopentenyloxypyrimidines in the preparation of pyrimidine carbonucleosides.
Falck-Pedersen ML, et al.
Acta Chemica Scandinavica, 47, 72-72 (1993)
596144
CorpBase ID (for auto-filling citation data) null
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service