141712
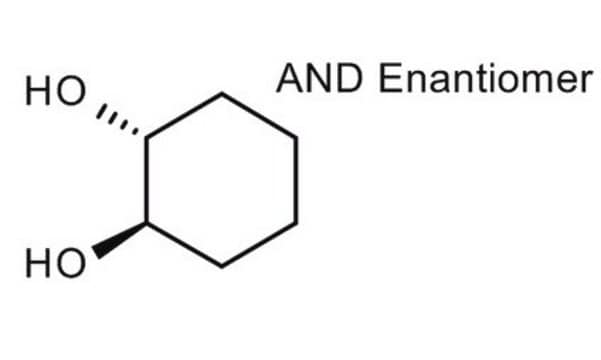
trans-1,2-Cyclohexanediol
98%
Synonym(s):
1,2-trans -Cyclohexanediol, 1,2-trans -Dihydroxycyclohexane, trans -2-Hydroxycyclohexanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein:
3193810
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
solid
mp
101-104 °C (lit.)
SMILES string
O[C@@H]1CCCC[C@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6-/m1/s1
InChI key
PFURGBBHAOXLIO-PHDIDXHHSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lisa D Cervia et al.
Molecular therapy. Nucleic acids, 11, 263-271 (2018-06-03)
The nuclear envelope is a physiological barrier to electrogene transfer. To understand different mechanisms of the nuclear entry for electrotransfected plasmid DNA (pDNA), the current study investigated how manipulation of the mechanisms could affect electrotransfection efficiency (eTE), transgene expression level
Jean Detry et al.
Applied microbiology and biotechnology, 72(6), 1107-1116 (2006-04-06)
Two extracellular lipases from Bacillus subtilis, B. subtilis lipase A and lipase B, have been expressed in the heterologous host Escherichia coli, biochemically characterized and used for the kinetic resolution of (rac)-trans-1,2-diacetoxycyclohexane. Both enzymes were selectively acting on the (R,R)-enantiomer
M Shahjahan Kabir et al.
The Journal of organic chemistry, 75(11), 3626-3643 (2010-05-01)
cis-1,2-Cyclohexanediol (L3) has been shown to be an efficient and versatile bidentate O-donor ligand that provides a highly active Cu-catalytic system. It was more effective than diols such as trans-1,2-cyclohexanediol or ethylene glycol. This commercially available cis-1,2-cyclohexanediol ligand facilitated the
M Tanaka et al.
The Journal of organic chemistry, 66(8), 2667-2673 (2001-04-17)
Diastereoselective alkylation of ethyl 2-methyl- and/or 2-ethylacetoacetates using the (S,S)-cyclohexane-1,2-diol as an acetal chiral auxiliary afforded enol ethers (2a-f and 5a-f) of 92->95% de in 31-70% yields. Removal of the cyclohexane-1,2-diol with BF(3)-OEt(2) afforded beta-keto esters (3 and 6) bearing
Roosmarijn E Vandenbroucke et al.
Nucleic acids research, 35(12), e86-e86 (2007-06-23)
One of the major obstacles in non-viral gene transfer is the nuclear membrane. Attempts to improve the transport of DNA to the nucleus through the use of nuclear localization signals or importin-beta have achieved limited success. It has been proposed
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service