F3648
Anti-Fibronectin antibody produced in rabbit
affinity isolated antibody, buffered aqueous solution
Synonym(s):
Fibronectin Antibody, Fibronectin Antibody - Anti-Fibronectin antibody produced in rabbit, Fibronectin Antibody Sigma
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
species reactivity
human
technique(s)
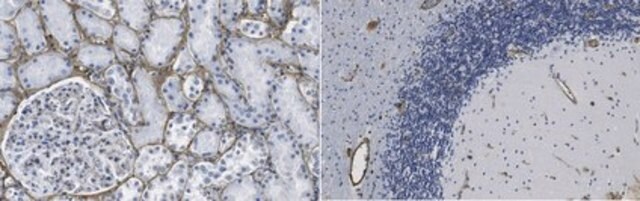
indirect ELISA: 1:10,000 using human fibronectin
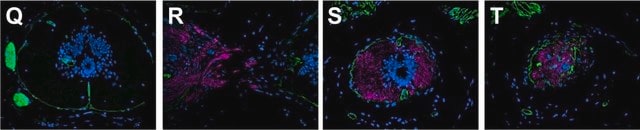
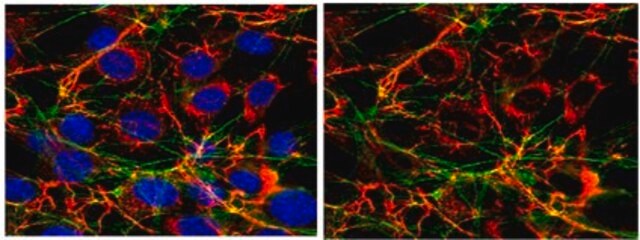
indirect immunofluorescence: 1:400 using human foreskin cultured fibroblasts
microarray: suitable
western blot: 1:1,000 using human plasma fibronectin
UniProt accession no.
application(s)
research pathology
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... FN1(2335)
General description
Fibronectin (FN) is composed of two nearly identical disulfide-bound polypeptides of molecular weight 220 kDa. Cellular fibronectin is structurally and antigenically similar to cold insoluble globulin from plasma, therefore polyclonal antibodies to either form usually cross-react. Careful analysis of the fibronectin molecule indicates that it contains several functionally and structurally distinct domains, which may bind to cell surfaces, collagen, fibrinogen or fibrin, complement, glycosaminoglycans, proteoglycans and heparin. Fibronectin 1 is encoded by the gene mapped to human chromosome 2q35.
Specificity
Immunogen
Application
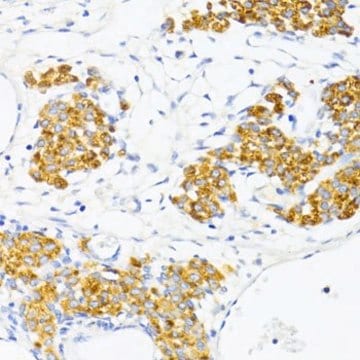
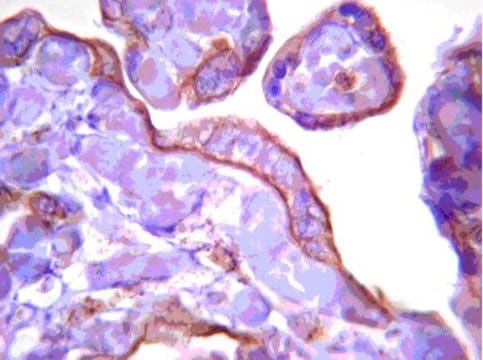
- immunohistochemistry (IHC) analysis to study the activity of pancreatic stellate cells in chronic pancreatitis
- antibody blocking assays of bacterial cells to study the activity of fibronectin in the Yersinia pestis binding to host cells and Yop delivery
- staining of endothelial cells as primary antibody
- cell adhesion assays to study the effect of anti-human fibronectin antibodies on adhesion to endothelial cells
- immunoprecipitation and western blot analysis of NF-κB dependent mammary tumorigenesis study
- immunofluorescence staining to study the correlation between zyxin tails and fibrillar adhesions
- immunoblotting to study the expression levels of the pancreatic stellate cells (PSC) markers glial fibrillary acidic protein (GFAP), α-smooth muscle actin (α-SMA), collagen type I, and fibronectin in whole cell lysates
- ELISA of fibronectin secretion
- microarray analysis.
Biochem/physiol Actions
Numerous studies have shown that fibronectin may enhance cell adhesion and spreading and affect the routes of cell migration both in vivo and in culture. Moreover, it has been shown that upon malignant transformation many cells lose most of their surface bound fibronectin. Fibronectin has been shown to also play a role in cellular morphology, cytoskeletal organization, phagocytosis, hemostasis, embryonic differentiation and wound repair. Fibronectin is produced by a wide variety of epithelial and mesenchymal cells in vitro including: fibroblasts, chondrocytes, myoblasts, Schwann cells, macrophages, hepatocytes and intestinal epithelial cells. Cellular fibronectin is present in many tissues including spleen, lymph node, tonsil, blood vessel walls, liver, kidney, muscle, skin, brain and peripheral nerves. It is found in basement membranes and in loose connective tissue stroma. It is also present in platelet α-granules and is expressed on the platelet surface after activation.
Physical form
Storage and Stability
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service