78825
Liquiritigenin
≥97.0% (HPLC)
Synonym(s):
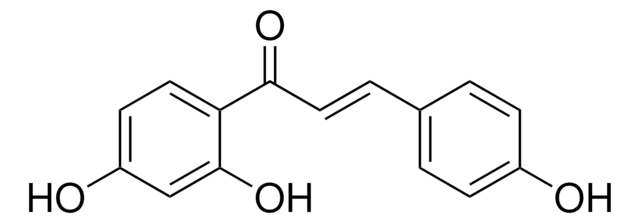
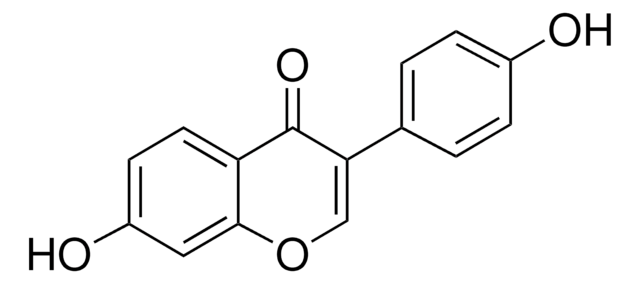
7,4′-Dihydroxyflavanone, 7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H12O4
CAS Number:
Molecular Weight:
256.25
Beilstein:
359378
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.28
Recommended Products
Quality Level
Assay
≥97.0% (HPLC)
form
powder or crystals
impurities
≤7% water
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
Oc1ccc(cc1)[C@@H]2CC(=O)c3ccc(O)cc3O2
InChI
1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1
InChI key
FURUXTVZLHCCNA-AWEZNQCLSA-N
General description
Liquiritigenin is a flavonoid and an estrogenic compound found in licorice (Glycyrrhizae radix) root extract and several other plants.
Application
Liquiritigenin has been used:
- to study its inhibitory effect on tumor metastasis in the treatment of colorectal cancer
- as a reference standard for ultra-performance liquid chromatography (UPLC) of Chaihu-Shugan-San (CSS) extract
- as a potential antiviral drug against hepatitis C virus (HCV) infection
Biochem/physiol Actions
Liquiritigenin displays anti-diabetic and choleretic properties. It exerts anti-inflammatory activity on Raw246.7 cells by inhibiting nuclear factor kappa light chain enhancer of activated B cells (NF-κB)-dependent-induction of inducible NOS (iNOS). Liquiritigenin inhibits liver fibrogenesis by blocking Hippo/Yes-associated protein (YAP) and transforming growth factor-β1 (TGF-β1)/small mothers against decapentaplegic (Smad) components. It is a selective estrogen receptor β agonist cells. Liquiritigenin induces apoptosis in SMM-721 cells by disruption of the mitochondrial membrane potential and increased production of reactive oxygen species.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rui Ting Liu et al.
European journal of pharmacology, 669(1-3), 76-83 (2011-08-30)
The present paper is to examine whether liquiritigenin is able to attenuate the Alzheimer's-like learning and memory deficits in a transgenic (Tg) mouse model that over-expresses amyloid protein precursor (APP), and explores the underlying mechanisms. Consistent with our previous observations
Eun-Ju Yang et al.
Neurotoxicology, 39, 114-123 (2013-09-10)
The progressive death of neurons following exposure to high concentrations of glutamate leads to loss of learning and memory and pathogenesis of neurodegenerative disorders. Therefore, identification of drugs that protect against glutamate-mediated neuronal cell death is a good strategy for
Suengmok Cho et al.
Bioorganic & medicinal chemistry, 20(11), 3493-3501 (2012-05-01)
Licorice (Glycyrrhiza glabra, GG) is one of the most frequently used herbal medicines worldwide, and its various biological activities have been widely studied. GG is reported to have neurological properties such as antidepressant, anxiolytic, and anticonvulsant effects. However, its hypnotic
Eun Mi Choi
International immunopharmacology, 12(1), 139-143 (2011-11-26)
Liquiritigenin is one of the flavonoids present in Glycyrrhizae radix. In the present study, the effects of liquiritigenin on the function of osteoblastic MC3T3-E1 cells were studied. Liquiritigenin caused a significant elevation of cell growth, alkaline phosphatase activity, collagen synthesis
Young Woo Kim et al.
American journal of physiology. Gastrointestinal and liver physiology, 296(2), G372-G381 (2008-12-17)
Liquiritigenin (LQ), an active component of licorice, has an inhibitory effect on LPS-induced inhibitory nitric oxide synthase expression. This study investigated the effects of LQ on choleresis, the expression of hepatic transporters and phase-II enzymes, and fulminant hepatitis. The choleretic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service