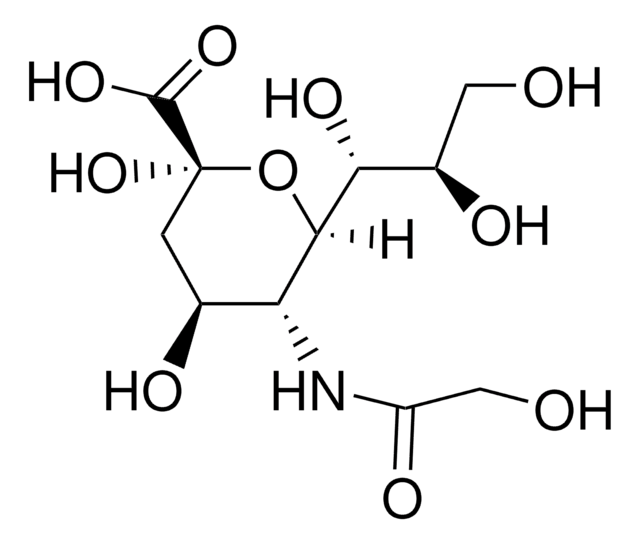
50644
N-Glycolylneuraminic acid
≥95% (HPLC)
Synonym(s):
Neu5Glc, NeuNGl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H19NO10
CAS Number:
Molecular Weight:
325.27
Beilstein:
1716828
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
Recommended Products
biological source
synthetic
Assay
≥95% (HPLC)
form
powder
storage temp.
−20°C
SMILES string
[H][C@]1(O[C@@](O)(C[C@H](O)[C@H]1NC(=O)CO)C(O)=O)[C@H](O)[C@H](O)CO
InChI
1S/C11H19NO10/c13-2-5(16)8(18)9-7(12-6(17)3-14)4(15)1-11(21,22-9)10(19)20/h4-5,7-9,13-16,18,21H,1-3H2,(H,12,17)(H,19,20)/t4-,5+,7+,8+,9+,11-/m0/s1
InChI key
FDJKUWYYUZCUJX-AJKRCSPLSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Glycolylneuraminic acid, a component of non-human milk and colostrums, is used as a reference to analyse changes of the glycome during lactation. Glycolylneuraminic acid, a xenoantigen, is involved in antigenic reactions (antibody-mediated hyperacute rejection) during xenotransplantion.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Regulation of N-glycolylneuraminic acid biosynthesis in rat and mouse liver
Sales restrictions may apply
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Lepers et al.
European journal of biochemistry, 193(3), 715-723 (1990-11-13)
The relative contribution of N-glycoloyl-beta-D-neuraminic acid (Neu5Gc) to total sialic acids expressed in mouse and rat liver glycoconjugates was found to be 95% and 11%, respectively. This considerable difference in sialic acid composition made these two tissues suitable models for
Jonas Löfling et al.
Virology, 440(1), 89-96 (2013-03-19)
Feline panleukopenia virus (FPV) is a pathogen whose canine-adapted form (canine parvovirus (CPV)) emerged in 1978. These viruses infect by binding host transferrin receptor type-1 (TfR), but also hemagglutinate erythrocytes. We show that hemagglutination involves selective recognition of the non-human
Alexandra S Gambaryan et al.
Journal of virology, 86(8), 4370-4379 (2012-02-22)
Influenza viruses of gallinaceous poultry and wild aquatic birds usually have distinguishable receptor-binding properties. Here we used a panel of synthetic sialylglycopolymers and solid-phase receptor-binding assays to characterize receptor-binding profiles of about 70 H7 influenza viruses isolated from aquatic birds
Jong-Yi Park et al.
Cellular reprogramming, 14(4), 353-363 (2012-07-11)
In this study, we examined whether Hanganutziu-Deicher (H-D) antigens are important as an immunogenic non-α1,3-galactose (Gal) epitope in pigs with a disrupted α1,3-galactosyltransferase gene. The targeting efficiency of the AO blood genotype was achieved (2.2%) in pig fibroblast cells. A
Rachel E Taylor et al.
The Journal of experimental medicine, 207(8), 1637-1646 (2010-07-14)
The nonhuman sialic acid N-glycolylneuraminic acid (Neu5Gc) is metabolically incorporated into human tissues from certain mammalian-derived foods, and this occurs in the face of an anti-Neu5Gc "xeno-autoantibody" response. Given evidence that this process contributes to chronic inflammation in some diseases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service