All Photos(1)
About This Item
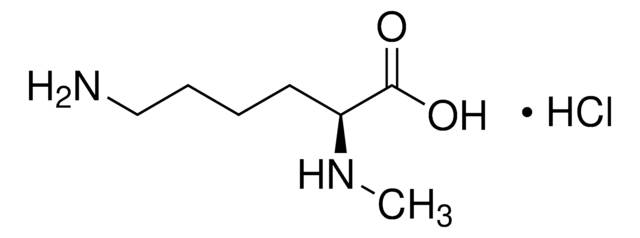
Empirical Formula (Hill Notation):
C7H16N2O2 · HCl
CAS Number:
Molecular Weight:
196.68
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥98.0% (TLC)
optical activity
[α]/D 20.5±1.5°, c = 0.1 in 1 M HCl
storage temp.
2-8°C
SMILES string
Cl.CNCCCC[C@H](N)C(O)=O
InChI
1S/C7H16N2O2.ClH/c1-9-5-3-2-4-6(8)7(10)11;/h6,9H,2-5,8H2,1H3,(H,10,11);1H/t6-;/m0./s1
InChI key
AQELUQTVJOFFBN-RGMNGODLSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
N ε-methyl-L-lysine was identified as a lysine analog with inhibitory effects on the growth and sporulation of Penicillium chrysogenum and benzyl-penicillin formation by mycelia.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matthew F Bush et al.
The journal of physical chemistry. A, 111(32), 7753-7760 (2007-07-20)
The gas-phase structures of protonated and alkali-metal-cationized lysine (Lys) and epsilon-N-methyllysine (Lys(Me)) are investigated using infrared multiple photon dissociation (IRMPD) spectroscopy utilizing light generated by a free electron laser, in conjunction with ab initio calculations. IRMPD spectra of Lys.Li(+) and
Duy P Nguyen et al.
Journal of the American Chemical Society, 131(40), 14194-14195 (2009-09-24)
Lysine methylation is an important post-translational modification of histone proteins that defines epigenetic status and controls heterochromatin formation, X-chromosome inactivation, genome imprinting, DNA repair, and transcriptional regulation. Despite considerable efforts by chemical biologists to synthesize modified histones for use in
Agnes M Móricz et al.
Natural product communications, 6(5), 657-660 (2011-05-28)
The influence of monomethylated basic amino acids [NG-monomethyl-L-arginine (MMA) and Nepsilon-monomethyl-L-lysine (MML)] and ozone capturers (indigo carmine, d-limonene) on the antibacterial effect of the mycotoxins aflatoxins B1, B2, G1 and G2 was studied in BioArena, which is a complex bioautographic
B Maras et al.
European journal of biochemistry, 203(1-2), 81-87 (1992-01-15)
The complete amino acid sequence of glutamate dehydrogenase from the thermoacidophilic archaebacterium Sulfolobus solfataricus has been determined. The sequence was reconstructed by automated sequence analysis of peptides obtained after cleavage by trypsin, cyanogen bromide, Staphylococcus aureus V8 protease and pepsin.
Methylated lysines and 3-methylhistidine in myosin: tissue and developmental differences.
G Huszar
Methods in enzymology, 106, 287-295 (1984-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service