D102504
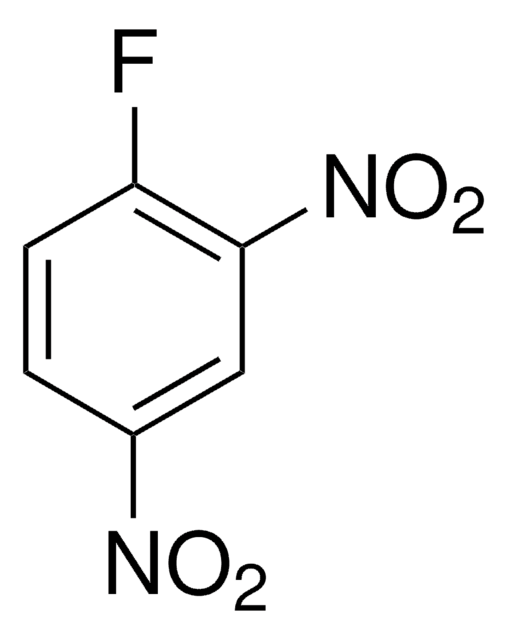
1,5-Difluoro-2,4-dinitrobenzene
97%
Synonym(s):
DFDNB
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H2(NO2)2
CAS Number:
Molecular Weight:
204.09
Beilstein:
1883116
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
72-74 °C (lit.)
SMILES string
[O-][N+](=O)c1cc(c(F)cc1F)[N+]([O-])=O
InChI
1S/C6H2F2N2O4/c7-3-1-4(8)6(10(13)14)2-5(3)9(11)12/h1-2H
InChI key
VILFTWLXLYIEMV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - STOT RE 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Suman Thummanagoti et al.
Molecular diversity, 15(1), 101-107 (2010-03-20)
Traceless synthesis of 2-aminoimidazoquinoxalinones has been performed on soluble polymer support under open-vessel microwave dielectric heating. The reaction progression is monitored directly by the conventional proton NMR which indicated no release of the substrate from the support. Fmoc-deprotected amino acid
Dong Mei Wang et al.
Journal of combinatorial chemistry, 11(4), 556-575 (2009-05-28)
This paper reports a versatile, good-yielding, solution-phase method that is a substituent diversity-directed synthesis of 1H-indoles (6-13, 17-20) and 1-hydroxyindoles (14, 15) starting from commercially available 1,5-difluoro-2,4-dinitrobenzene. The synthetic products possessed the maximum six diversity points.
Gang Liu et al.
Journal of combinatorial chemistry, 9(1), 70-78 (2007-01-09)
This paper describes our recent efforts to synthesize novel compound scaffolds integrating 2-quinoxalinol with privileged structures of 1,3-dihydro-benzoimidazol-2-one, 1,3-dihydro-benzoimidazole-2-thione, 3-hydroxy-1H-quinoxalin-2-one, 2H-benzo[1,4]oxazin-3-ol, 2H-benzo[1,4]thiazin-3-ol, and 1,3,4,5-tetrahydro-benzo[1,4]diazepin-2-one, respectively. Eight novel benzofused tricycles and their substituent diversity points were developed. These include pyrazino[2,3-g]quinoxaline-2,8-diol (I)
S M Hourani et al.
General pharmacology, 20(4), 413-416 (1989-01-01)
1. The effects of some possible inhibitors of ectonucleotidases on the breakdown of extracellular ATP by strips of guinea-pig urinary bladder were investigated. 2. Suramin and ethacrynic acid (10 mM) both inhibited ATP breakdown significantly, and difluorodinitrobenzene (10 mM) inhibited
M R Ermácora et al.
International journal of peptide and protein research, 30(3), 423-430 (1987-09-01)
Derivatives of bovine growth hormone, containing monoaminotyrosyl residues in positions 35, 42 and 174, were treated at pH 3.6 with a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Under these conditions aminotyrosyl groups reacted. On changing the pH to 9.3, the second fluorine atom
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)






