927090
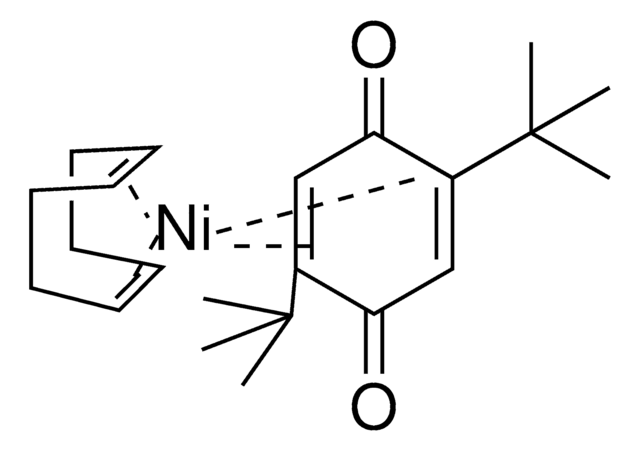
Ni(COD)(CPDO-(OMe)Ph)
≥95%
Synonym(s):
(1,5-cyclooctadiene)(2,3,4,5-tetrakis(4-methoxyphenyl)cyclopenta-2,4-dien-1-one)nickel(0)
Select a Size
CHF 21.00
Availability
Estimated to ship onFebruary 22, 2025
Select a Size
About This Item
CHF 21.00
Availability
Estimated to ship onFebruary 22, 2025
Recommended Products
Quality Level
Assay
≥95%
form
powder or chunks
reaction suitability
reagent type: catalyst
reaction type: Cross Couplings
mp
>300 °C
storage temp.
2-8°C
Application
Reference
Structurally Diverse Bench-Stable Nickel(0) Pre-Catalysts: A Practical Toolkit for In Situ Ligation Protocols
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service