All Photos(1)
About This Item
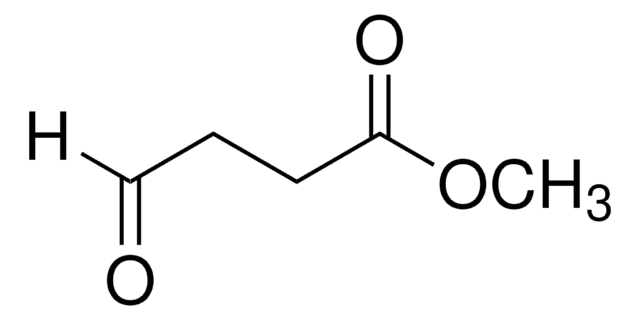
Linear Formula:
(CH3O)2CH(CH2)3CO2CH3
CAS Number:
Molecular Weight:
176.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.422 (lit.)
bp
70-72 °C/2 mmHg (lit.)
density
1.012 g/mL at 25 °C (lit.)
functional group
acetal
ester
ether
SMILES string
COC(CCCC(=O)OC)OC
InChI
1S/C8H16O4/c1-10-7(9)5-4-6-8(11-2)12-3/h8H,4-6H2,1-3H3
InChI key
YOFAONQHOIRLCQ-UHFFFAOYSA-N
Related Categories
General description
Methyl 5,5-dimethoxyvalerate (methyl 5,5-dimethoxypentanoate) is an ester. It can be prepared by reacting methyl 5-oxopentanoate with p-toluene sulfonic acid and trimethylorthoformate. It participates in the synthesis of 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine.
Application
Methyl 5,5-dimethoxyvalerate may be employed in the synthesis of seven-membered carbocycles. It may be used in the synthesis of 5-(phenylamino)-4-(phenylimino)methyl)-4-pentenoic acid derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Formation of seven-membered carbocycles by the use of cyclopropyl silyl ethers as homoenols.
Oleg L Epstein et al.
Angewandte Chemie (International ed. in English), 45(30), 4988-4991 (2006-07-05)
Sanjay Srivastava et al.
The Journal of biological chemistry, 279(51), 53395-53406 (2004-10-07)
Oxidation of unsaturated phospholipids results in the generation of aldehyde side chains that remain esterified to the phospholipid backbone. Such "core" aldehydes elicit immune responses and promote inflammation. However, the biochemical mechanisms by which phospholipid aldehydes are metabolized or detoxified
Fangwei Shao et al.
Bioconjugate chemistry, 19(12), 2487-2491 (2008-12-05)
A facile synthetic route to prepare monofunctional carbocyanine dyes for biological application is developed. Three pentamethine carbocyanine dyes have been successfully modified with a variety of functional groups such as: carboxylic acids, azides, or alkynes. The new dyes are characterized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service