All Photos(2)
About This Item
Empirical Formula (Hill Notation):
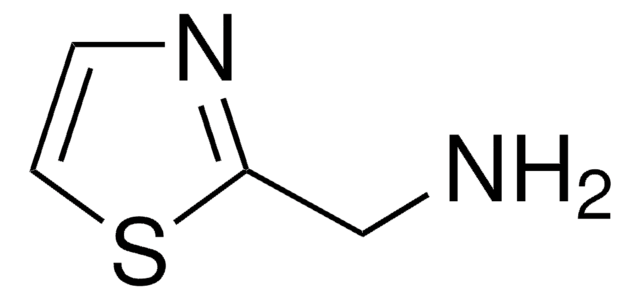
C4H6N2S
CAS Number:
Molecular Weight:
114.17
Beilstein:
109603
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
93-98 °C (lit.)
SMILES string
Cc1cnc(N)s1
InChI
1S/C4H6N2S/c1-3-2-6-4(5)7-3/h2H,1H3,(H2,5,6)
InChI key
GUABFMPMKJGSBQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-5-methylthiazole is a heterocyclic building block. It is one of the major alkaline metabolite of tenoxicam (TX) and meloxicam (MX).
Application
2-Amino-5-methylthiazole may be used in the preparation of acrylamide monomer, 5-methyl-2-thiozyl methacrylamide (MTMAAm).
2-Amino-5-methylthiazole may be used in the preparation of mixed-ligand dien-Cu(II) complexes (dien=diethylenetriamine). It may be used in the preparation of a new series of Schiff mono/dibase coordination compounds, having potential anticancer and antibacterial activities.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Riccardo Baron et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 9(7), 983-988 (2008-04-18)
The binding of 2-amino-5-methylthiazole to the W191G cavity mutant of cytochrome c peroxidase is an ideal test case to investigate the entropic contribution to the binding free energy due to changes in receptor flexibility. The dynamic and thermodynamic role of
A Th Chaviara et al.
Journal of inorganic biochemistry, 98(8), 1271-1283 (2004-07-24)
A new series of coordination compounds of the starting materials [Cu(dienX(2)Y(2))] and their adducts [Cu(dienXXY(2))(2a-5mt)] (where dien=diethylenetriamine, dienXX=Schiff bases of diethylenetriamine with 2-furaldehyde or 2-thiophene-carboxaldehyde, X=O, S, Y=Cl, Br, NO(3) and 2a-5mt=2-amino-5-methylthiazole) were synthesized by stepwise reactions and their structures
A Th Chaviara et al.
Journal of inorganic biochemistry, 99(2), 467-476 (2004-12-29)
Two novel mononuclear Cu(II) coordination compounds of the type [Cu(dptaS)Cl(2)] and [Cu(dptaS)Br(2)] (dptaS=1,3-propanediamine, N(1)-[3-aminopropyl]-N(3)-[2-thienylmethylidene] or Schiff mono-base of dipropylenetriamine with 2-thiophene-carboxaldehyde) were prepared by the hydrolysis of the di-bases, [Cu(dptaSS)Cl(2)] and [Cu(dptaSS)Br(2)] (dptaSS=1,3-propanediamine, N(1)-[2-thienylmethylidene]-N(3)-[[2-thienylmethylidene]aminopropyl] or Schiff di-base of dipropylenetriamine with
C A Bolos et al.
Journal of inorganic biochemistry, 88(1), 25-36 (2001-12-26)
The reaction of [Cu(dien)NO(3)]NO(3) with 2-amino-5-methylthiazole (2A5MT), 2-amino-2-thiazoline (2A-2Tzn), imidazole (im), N,N'-thiocarbonyldiimidazole (Tcdim), 2-aminothiazole (2AT) and 2-ethylimidazole (2Etim), gave a new series of mixed-ligand compounds of the general formula [Cu(dien)(B)NO(3))]NO(3); (dien, diethylenetriamine; B, 2A5MT, 2A-2Tzn, im, Tcdim, 2AT and 2Etim).
Ratio derivative spectrophotometric method for the determination of some oxicams in presence of their alkaline degradation products.
Taha EA, et al.
Scientia Pharmaceutica, 71(4), 303-320 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service