325104
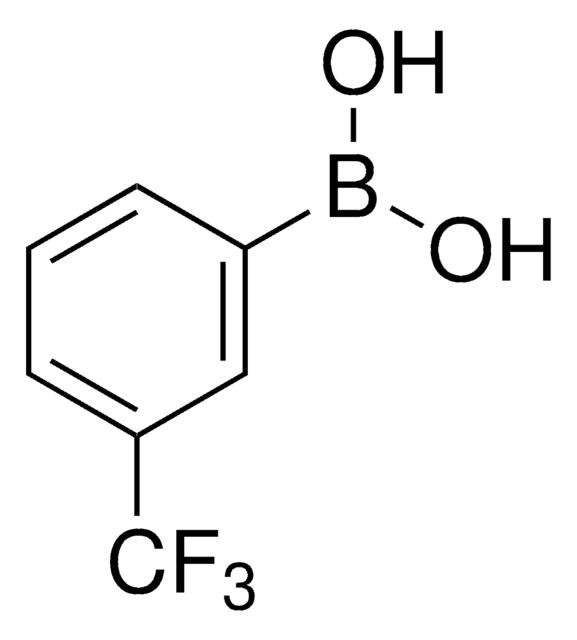
3-Nitrophenylboronic acid
≥97%
Synonym(s):
3-Nitrobenzeneboronic acid, m-Nitrobenzeneboronic acid, m-Nitrophenylboronic acid, NSC 401539, NSC 59739
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2NC6H4B(OH)2
CAS Number:
Molecular Weight:
166.93
Beilstein:
2938638
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
form
powder
mp
284-285 °C (dec.) (lit.)
functional group
nitro
SMILES string
OB(O)c1cccc(c1)[N+]([O-])=O
InChI
1S/C6H6BNO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,9-10H
InChI key
ZNRGSYUVFVNSAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Catalyzes ene carbocyclization of acetylenic dicarbonyl compounds
Reactant involved in:
Additionally used as a reactant for synthesizing biologically active molecules such as:
- Copper-catalyzed arylation
- Palladium-catalyzed decarboxylative coupling
- Suzuki-Miyaura cross-coupling
- Oxidative carbocyclization / arylation
- Addition to arylpropargyl alcohols
Additionally used as a reactant for synthesizing biologically active molecules such as:
- Inhibitors of angiogenesis
- Biaryl-olefins with antiproliferative activities
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Meiling Li et al.
Chemical communications (Cambridge, England), 46(13), 2191-2193 (2010-03-18)
The discovery and development of an efficient ene carbocyclization of 1,3-dicarbonyl compounds bearing pendent terminal alkyne substituents under 3-nitrobenzeneboronic acid catalysis is described. The reaction is efficient, easy to perform and general to a wide range of ketoester substrates.
Chiaki Miyamoto et al.
Inorganic chemistry, 47(5), 1417-1419 (2008-02-12)
The rate constants for a boronate ion were determined for the first time using the reaction systems of 3-nitrophenylboronic acid (3-NO2PhB(OH)2) with ethylene glycol (EG) and propylene glycol (PG) in an alkaline solution: the rate constants (25 degrees C, I
Maria T Gallardo-Williams et al.
The Prostate, 54(1), 44-49 (2002-12-14)
Prostate specific antigen (PSA) is a well-established marker of prostate cancer, but it can also degrade extracellular matrix proteins such as fibronectin and could be involved in tumor progression and metastasis. In this study, we have addressed the use of
B J Johnson
Biochemistry, 20(21), 6103-6108 (1981-10-13)
Highly purified isoaccepting species of transfer ribonucleic acid (tRNA) were prepared by use of a polyacrylamide substituted with nitrobenzeneboronic acid functional groups. This method exploits the well-known ability of boronic acids to complex with RNA cis-diols. tRNA isoacceptors were obtained
K C Usher et al.
Biochemistry, 37(46), 16082-16092 (1998-11-18)
The structures of AmpC beta-lactamase from Escherichia coli, alone and in complex with a transition-state analogue, have been determined by X-ray crystallography. The native enzyme was determined to 2.0 A resolution, and the structure with the transition-state analogue m-aminophenylboronic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service