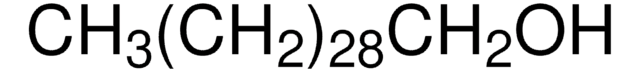All Photos(1)
About This Item
Linear Formula:
CH3(CH2)21OH
CAS Number:
Molecular Weight:
326.60
Beilstein:
1770470
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
180 °C/0.22 mmHg (lit.)
mp
65-72 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCCCCCCO
InChI
1S/C22H46O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23/h23H,2-22H2,1H3
InChI key
NOPFSRXAKWQILS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Docosanol inhibits replication of certain viruses (herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. It has been isolated from Clematis brevicaudata.
Application
1-Docosanol was used in the synthesis of series of amphiphilic dendrimers with hydrophilic aliphatic polyether-type dendritic core and hydrophobic docosyl peripheries.
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
410.0 °F
Flash Point(C)
210 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and self-assembly of amphiphilic dendrimers based on aliphatic polyether-type dendritic cores.
Cho B-K, et al.
Macromolecules, 37(11), 4227-4234 (2004)
Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex.
D H Katz et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10825-10829 (1991-12-01)
This article reports that 1-docosanol, a 22-carbon-long saturated alcohol, exerts a substantial inhibitory effect on replication of certain viruses (e.g., herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. To study the basis for its viral
Guadalupe Iglesias et al.
Regulatory toxicology and pharmacology : RTP, 36(1), 80-85 (2002-10-18)
Behenyl alcohol is a saturated 22-carbon, long-chain aliphatic alcohol, which has potential for use in foods as an oil-structuring and -solidifying agent in fats. Previously completed studies with behenyl alcohol indicated an absence of mutagenic or genotoxic potential. In addition
Diogo C Morelli et al.
Journal of chromatography. A, 1626, 461377-461377 (2020-08-17)
This study reports the use ofa natural deep eutectic solvent (NADES) with hollow fiber-microporous membrane liquid-liquid microextraction (HF-MMLLE) for the multiclass determination of 11 compounds classified as emerging contaminantsin water. Different deep eutectic solvents were synthetized and Thymol: Camphor (1:1
John F Marcelletti
Antiviral research, 56(2), 153-166 (2002-10-09)
Interactions between docosanol (n-docosanol, behenyl alcohol) and nucleoside or pyrophosphate analogs were investigated in vitro. The anti-HSV activity of acyclovir (ACV) was synergistically enhanced by treatment of cells with docosanol as judged by inhibition of progeny virus production and plaque
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service