122750
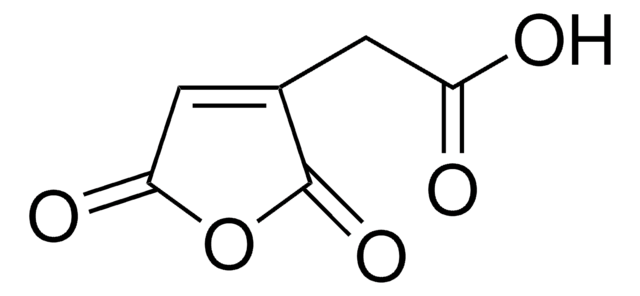
trans-Aconitic acid
98%
Synonym(s):
trans-Propene-1,2,3-tricarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HO2CCH2C(CO2H)=CHCO2H
CAS Number:
Molecular Weight:
174.11
Beilstein:
1725830
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
190 °C (dec.) (lit.)
SMILES string
OC(=O)C\C(=C/C(O)=O)C(O)=O
InChI
1S/C6H6O6/c7-4(8)1-3(6(11)12)2-5(9)10/h1H,2H2,(H,7,8)(H,9,10)(H,11,12)/b3-1+
InChI key
GTZCVFVGUGFEME-HNQUOIGGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
trans-Aconitic acid is inhibitor of the tricarboxylic acid cycle and was identified in range grasses.
trans-Aconitic acid is an antioxidant used in food and cosmetics preparation and also used as a plasticizer in the polymer industry.
trans-Aconitic acid is an antioxidant used in food and cosmetics preparation and also used as a plasticizer in the polymer industry.
Application
trans-Aconitic acid was used in synthesis of aconitate esters. It may be used for cross-linking polyvinyl alcohol film for preparing composite nanofiltration membranes.
Biochem/physiol Actions
Antimicrobial agent that blocks the transformation of Leishmania donovani amastigotes to prostigotes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Quantitation of trans-aconitic acid in different stages of the sugar-manufacturing process
Montoya, Guillermo and Londono, et al.
Journal of Agricultural and Food Chemistry, 62, 8314-8831 (2014)
R Burau et al.
Science (New York, N.Y.), 150(3697), 766-767 (1965-11-05)
trans-Aconitate ion, an inhibitor of the tricarboxylic acid cycle, was identified in range grasses as trans-aconitic acid, which was isolated in crystalline form. It occurs in surprisingly high concentrations in early-season forage grasses. Dry-weight concentrations of trans-aconitate vary with season
Effect of catalytic conditions on the synthesis of new aconitate esters.
Piang-Siong W, et al.
Industrial Crops and Products, 35(1), 203-210 (2012)
Effect of Cross-Linking Agent Chemistry and Coating Conditions on Physical, Chemical and Separation Properties of PVA-PSf Composite Membranes.
Dlamini DS, et al. et al.
Separation Science and Technology (2013)
Jiayin Pang et al.
Physiologia plantarum, 154(4), 511-525 (2014-10-08)
The aim of this study was to investigate the capacity of three perennial legume species to access sources of varyingly soluble phosphorus (P) and their associated morphological and physiological adaptations. Two Australian native legumes with pasture potential (Cullen australasicum and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service