10879
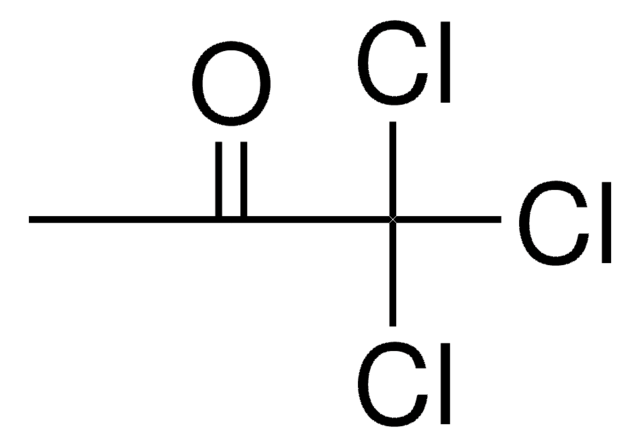
1,1,3-Trichloroacetone
Wacker Chemie AG, ≥86.5% (GC)
Synonym(s):
TCA, 1,1,3-Trichloro-2-propanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(Cl)2CHCOCH2Cl
CAS Number:
Molecular Weight:
161.41
Beilstein:
1746647
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥86.5% (GC)
manufacturer/tradename
Wacker Chemie AG
bp
88-90 °C/76 mmHg (lit.)
mp
9-11 °C (lit.)
density
1.512 g/mL at 20 °C (lit.)
functional group
chloro
ketone
SMILES string
ClCC(=O)C(Cl)Cl
InChI
1S/C3H3Cl3O/c4-1-2(7)3(5)6/h3H,1H2
InChI key
ZWILTCXCTVMANU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,1,3-Trichloroacetone is direct-acting mutagens in the Ames/Salmonella assay. It is precursor for the generation of 1,3-dihalo oxyallyl intermediates, which undergo [4 + 3] cycloadditions with a host of 1,3-diene systems.
Application
1,1,3-Trichloroacetone was used in base-induced solvolyses of [3.2.1]Bicyclic α,α′-dichloro ketones.
Other Notes
prices for bulk quantities on request
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1B
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Base-Induced Solvolyses of [3.2. 1] Bicyclic a, a'-Dichloro Ketones- 1, 3-Transposition and Ring-Contraction.
Fohlisch B, et al.
European Journal of Organic Chemistry, 2001(22), 4357-4365 (2001)
W F Blazak et al.
Mutation research, 206(4), 431-438 (1988-12-01)
1,1,1- and 1,1,3-trichloroacetones (TCA) result from the disinfection of municipal water supplies with chlorine, and are direct-acting mutagens in the Ames/Salmonella assay. The objective of this study was to further investigate the genotoxicity of these compounds in mammalian cells using
Ho Lam Chan et al.
Nature communications, 9(1), 3377-3377 (2018-08-25)
Polycomb repressive complex 1 (PRC1) plays essential roles in cell fate decisions and development. However, its role in cancer is less well understood. Here, we show that RNF2, encoding RING1B, and canonical PRC1 (cPRC1) genes are overexpressed in breast cancer.
M Robinson et al.
Cancer letters, 48(3), 197-203 (1989-12-01)
Several chlorinated acetones have been identified in drinking water and these, as well as a number of chlorinated acroleins, are produced by chlorination of humic acid solutions. Many of these chlorinated compounds and the brominated acrolein analog were positive in
Yusheng Zhang et al.
Science advances, 6(23), eaaz7249-eaaz7249 (2020-06-18)
RING1B, a core Polycomb repressive complex 1 subunit, is a histone H2A ubiquitin ligase essential for development. RING1B is overexpressed in patients with luminal breast cancer (BC) and recruited to actively transcribed genes and enhancers co-occupied by the estrogen receptor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service