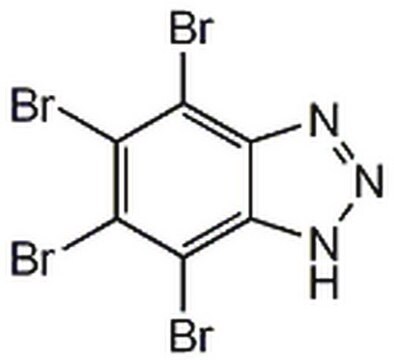
T6951
TBBz
≥98% (HPLC), powder
Synonym(s):
4,5,6,7-Tetrabromobenzimidazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H2N2Br4
CAS Number:
Molecular Weight:
433.72
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
solubility
DMSO: >10 mg/mL at 60 °C, clear
storage temp.
2-8°C
SMILES string
Brc1c(Br)c(Br)c2[nH]cnc2c1Br
InChI
1S/C7H2Br4N2/c8-2-3(9)5(11)7-6(4(2)10)12-1-13-7/h1H,(H,12,13)
InChI key
LOEIRDBRYBHAJB-UHFFFAOYSA-N
Application
TBBz has been used as a CK2 inhibitor in HeLa cells and rat septal neurons.
Biochem/physiol Actions
TBBz is a cell-permeable Casein Kinase-2 (CK2) inhibitor. CK2 inhibitors, 4,5,6,7-tetrabromobenzotriazole (TBBt, Sigma Cat. # T0826) and rabromobenzimidazole (TBBz), the latter of which was shown to discriminate between different molecular forms of CK2 in yeast. TBBt, with a pK(a) ~5, exists in solution at physiological pH almost exclusively (>99%) as the monoanion; whereas TBBz, with a pKa ~9, is predominantly (>95%) in the neutral form, both of obvious relevance to their modes of binding. In vitro, TBBt inhibits different forms of CK2 with Ki values ranging from 80 to 210 nM. TBBz discriminates better between CK2 forms, with Ki values ranging from 70-510 nM. TBBz is more effective than TBBt in inducing apoptosis and to a lesser degree, necrosis in transformed human cell lines. Dvelopment of shRNA strategies for the selective knockdown of the CK2α and CK2α′ isoforms reinforces the foregoing results, indicating that inhibition of CK2 leads to attenuation of proliferation.
Preparation Note
TBBz is soluble in DMSO (60 deg C) at a concentration that is greater than 10 mg/ml.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Florence Chaverneff et al.
Journal of neurochemistry, 109(3), 733-743 (2009-02-19)
The combination of bone morphogenetic protein 7 (BMP7) and neurotrophins (e.g. brain-derived neurotrophic factor, BDNF) protects septal neurons during hypoglycemic stress. We investigated the signaling mechanisms underlying this synergistic protection. BMP7 (5 nM) increased phosphorylation and nuclear translocation of BMP-responsive
Maria Russo et al.
Oncotarget, 8(26), 42571-42587 (2017-05-11)
Despite the encouraging results of the innovative therapeutic treatments, complete remission is uncommon in patients affected by chronic lymphocytic leukaemia, which remains an essentially incurable disease. Recently, clinical trials based on BH3-mimetic drugs showed positive outcomes in subjects with poor
Jacob P Turowec et al.
Oncotarget, 4(4), 560-571 (2013-04-20)
Protein kinase CK2 has emerged as a promising candidate for the treatment of a number of cancers. This enzyme is comprised of two catalytic subunits (CK2 and/or CK2α') that form complexes with homodimers of regulatory CK2β subunits. While catalytic and
James S Duncan et al.
Molecular & cellular proteomics : MCP, 7(6), 1077-1088 (2008-02-09)
Recently protein kinases have emerged as some of the most promising drug targets; and therefore, pharmaceutical strategies have been developed to inhibit kinases in the treatment of a variety of diseases. CK2 is a serine/threonine-protein kinase that has been implicated
Laszlo Gyenis et al.
Journal of proteome research, 10(11), 4887-4901 (2011-09-23)
Protein kinases have emerged as attractive targets for treatment of several diseases prompting large-scale phosphoproteomics studies to elucidate their cellular actions and the design of novel inhibitory compounds. Current limitations include extensive reliance on consensus predictions to derive kinase-substrate relationships
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service