SRP0292
Cathepsin S Active human
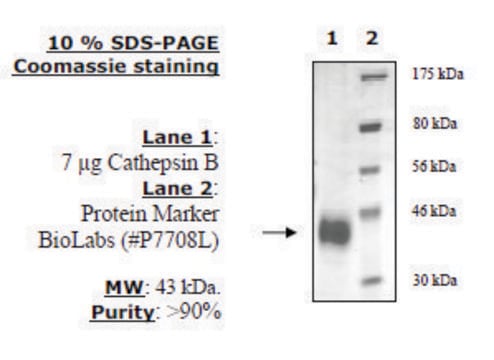
recombinant, expressed in FreeStyle™ 293-F cells, ≥90% (SDS-PAGE)
Synonym(s):
CTSS, MGC3886
About This Item
Recommended Products
biological source
human
recombinant
expressed in FreeStyle™ 293-F cells
Assay
≥90% (SDS-PAGE)
form
aqueous solution
specific activity
≥8100 pmol/min-μg
mol wt
37 kDa
technique(s)
activity assay: suitable
suitability
suitable for molecular biology
NCBI accession no.
application(s)
life science and biopharma
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... CTSS(1520)
General description
Human cathepsin S (GenBank Accession No. NM_004079.3), CD33 signal peptide (amino acids 1-16) + cathepsin S (amino acids 17-331), with C-terminal HIS tag, MW = 37 kDa, expressed in FreeStyle 293-F cells. Cathepsin S belongs to the cysteine cathepsin protease family. It has limited tissue expression, being associated with antigen-presenting cells localized in lymph and spleen, as well as other immune cells like macrophages. Human cathepsin S is produced from its corresponding CTSS gene on chromosome 1q21 and is synthesized as a pre-proenzyme.
Application
- to assess the pathogenesis of Alzheimer′s disease.
- to investigate the optimization of selectivity of Azepanone-based inhibitors.
- for the immunocytochemical detection of cathepsin-S in mouse samples.
- for incubating mouse brain sections to test the impact of cathepsin-S on perineuronal nets (PNNs) integrity.
Biochem/physiol Actions
Unit Definition
Physical form
Preparation Note
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service