SML3577
MMG-11
≥98% (HPLC)
Synonym(s):
Ethyl 5-(2-oxo-2-(2,3,4-trihydroxyphenyl)ethyl)furan-2-carboxylate, MMG 11, MMG11
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H14O7
CAS Number:
Molecular Weight:
306.27
MDL number:
UNSPSC Code:
51111800
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
, white to very dark gray
solubility
DMSO: 2 mg/mL, clear
storage temp.
2-8°C
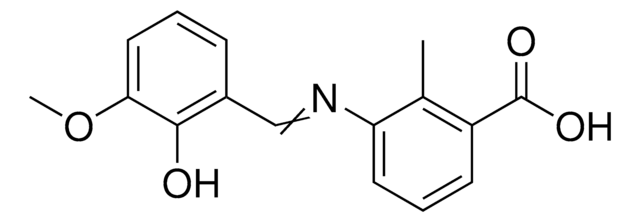
SMILES string
O=C(OCC)C1=CC=C(O1)CC(C2=CC=C(C(O)=C2O)O)=O
Biochem/physiol Actions
MMG-11 is a toll-like receptor 2 (TLR2) antagonist that selectively blocks TLR2/1 over TLR2/6 heterodimer-mediated sginaling in both human & murine species (NF-κB/AP-1 activation IC50 = 0.87 µM vs. 7.4 µM in hTLR2/1 or hTLR2/6 HEK293 reporter cells stimulated with 10 ng/mL TLR2/1 agonist Pam3CSK4 or 1 ng/mL TLR2/6 agonist Pam2CSK4; TNF/IL-6 secretion IC50 = 3.3/0.19 µM against 10 ng/mL Pam3CSK4 vs. 58/14 µM against 1 ng/mL Pam2CSK4 using murine macrophage RAW 264.7). In contrast, the TLR2 antagonist CU-CPT22 is selective for hTLR2/6 over hTLR2/1, while being weakly selective for mTLR2/1 over mTLR2/6 heterodimer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Identification of a pyrogallol derivative as a potent and selective human TLR2 antagonist by structure-based virtual screening
Biochemical Pharmacology, 154, 148-160 (2018)
Maria Grabowski et al.
Biochemical pharmacology, 171, 113687-113687 (2019-11-05)
Toll-like receptor 2 (TLR2) forms heterodimers with either TLR1 or TLR6 to induce protective early inflammatory responses to pathogen- and damage-associated molecular patterns. However, excessive activation is associated with inflammatory and metabolic diseases. Several TLR2 antagonists have been described but
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service