SML0610
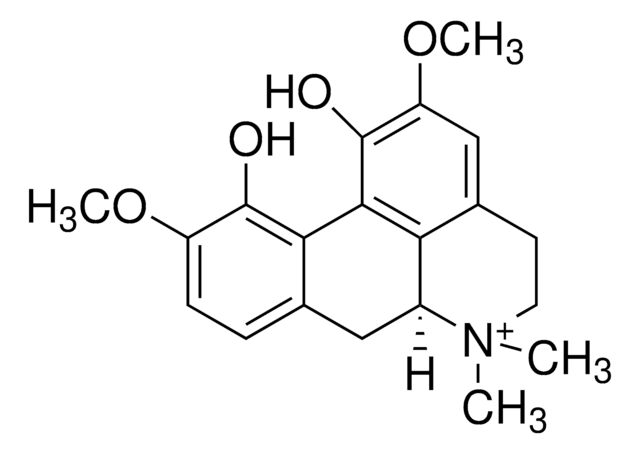
Nitidine chloride
≥97% (HPLC)
Synonym(s):
2,3-Dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridinium chloride, NSC 146397
About This Item
Recommended Products
Assay
≥97% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 1 mg/mL, clear (warmed)
shipped in
wet ice
storage temp.
−20°C
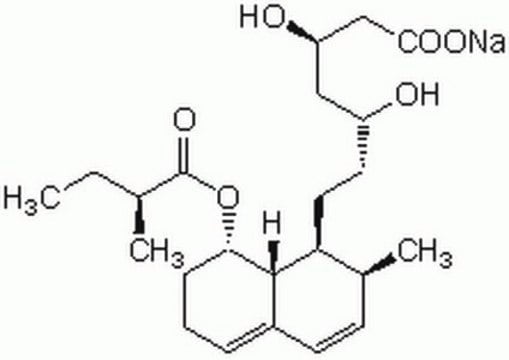
SMILES string
C[N+]1=CC2=CC(OC)=C(OC)C=C2C3=CC=C4C=C5C(OCO5)=CC4=C31.[Cl-]
InChI
1S/C21H18NO4.ClH/c1-22-10-13-7-17(23-2)18(24-3)8-15(13)14-5-4-12-6-19-20(26-11-25-19)9-16(12)21(14)22;/h4-10H,11H2,1-3H3;1H/q+1;/p-1
InChI key
QLDAACVSUMUMOR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The focal adhesion kinase (FAK) is a cytoplasmic protein tyrosine kinase. FAK has been implicated as a downstream signaling molecule that functions in the control of several integrin-regulated biological processes.
Agents reported to activate cellular caspases include chemotherapeutic drugs, TNF receptor agonists, and other enzymes. Inhibitors of apoptosis were the first identified endogenous caspase inhibitors.
Src is the original member of a group of non-receptor protein-tyrosine kinases termed the Src family kinases (SFKs). Learn about the SFKs that, in humans, consist of eight members, a small group of atypical members, and two related kinases.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![Dibenzo[a,l]pyrene BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/937/930/3e2321b0-d54a-46c2-bb84-007bb57eb381/640/3e2321b0-d54a-46c2-bb84-007bb57eb381.png)