MABN826
Anti-phospho-α-Synuclein (Ser129) Antibody, clone 81A
clone 81A, from mouse
Synonym(s):
Alpha-synuclein, NACP, Non-A beta component of AD amyloid, Non-A4 component of amyloid precursor, Synuclein alpha-140
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
81A, monoclonal
species reactivity
mouse, human
technique(s)
electron microscopy: suitable
immunocytochemistry: suitable
immunofluorescence: suitable
immunohistochemistry: suitable (paraffin)
western blot: suitable
isotype
IgG2aκ
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
phosphorylation (pSer129)
Gene Information
human ... SNCA(6622)
General description
Specificity
Immunogen
Application
Immunocytochemistry Analysis: A representative lot detected Ser129-phosphorylated α-synuclein in Lewy bodies-/LB- and Lewy-neurite-/LN-like inclusions in cultured embryonic hippocampal neurons from wild-type mice and mice carrying human mutant P301S tau transgene, but not Snca-/- mice, upon exposure to preformed α-synuclein fibrils (pffs) from C-terminally truncated, Myc-tagged α-synuclein (Guo, J.L., and Giasson, B.I. (2013). Cell. 154(1):103-117).
Electron Microscopy Analysis: A representative lot detected phosphorylated α-synuclein in close physical associations of tau in filamentous structures within neuronal processes of human P301S mutant tau transgenic mouse embryo hippocampal neurons exposed to α-synuclein fibrils formed by repeated rounds of self-seeding using C-terminally truncated, Myc-tagged α-synuclein (Guo, J.L., and Giasson, B.I. (2013). Cell. 154(1):103-117).
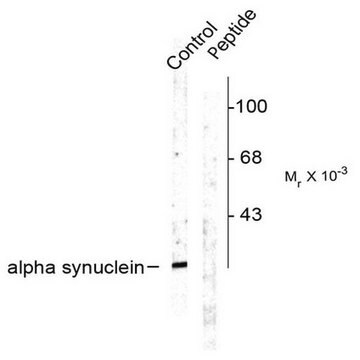
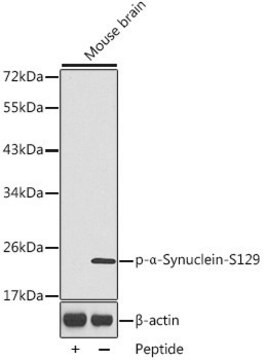
Western Blotting Analysis: A representative lot detected phosphorylation of the Triton-insoluble α-synuclein formed in cultured mouse embryonic hippocampal neurons upon exposure to preformed α-synuclein fibrils (pffs) from C-terminally truncated, Myc-tagged α-synuclein (Guo, J.L., and Giasson, B.I. (2013). Cell. 154(1):103-117).
Western Blotting Analysis: A representative lot detected Ser129 phosphorylation of Triton-insoluble, but not Triton-soluble, α-synuclein in the cingulate cortex extracts from DLB (dementia with Lewy bodies) patients and the cerebella extracts from patients with multiple systems atrophy (MSA) (Waxman, E.A., et al. (2008). J. Neuropathol. Exp. Neurol. 67(5):402-416).
Western Blotting Analysis: A representative lot detected CK1- and CK2-catalyzed alpha-synuclein Ser129 phosphorylation, but not CK1-catalyzed α-synuclein Ser87 phosphorylation, nor non-phosphorylated α-synuclein (Waxman, E.A., et al. (2008). J. Neuropathol. Exp. Neurol. 67(5):402-416).
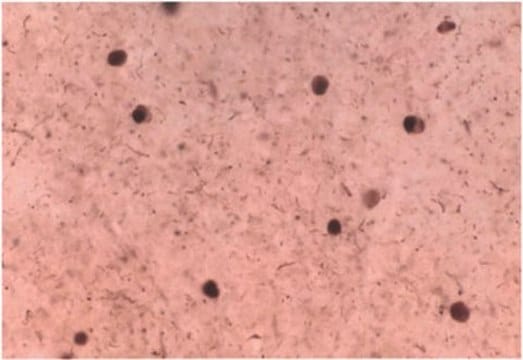
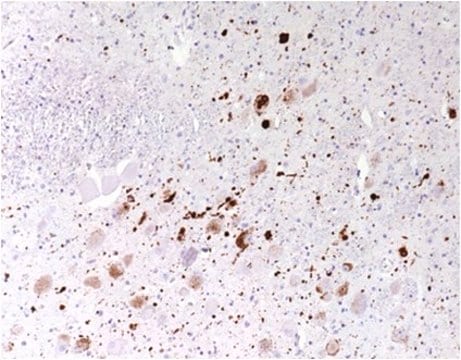
Immunohistochemistry Analysis: A representative lot detected pathologic inclusions-associated α-synuclein Ser129 phosphorylation in paraffin-embedded brain tissue sections from patients with PD (Parkinson′s disease), DLB (dementia with Lewy bodies) and MSA (multiple systems atrophy), while clone 81A detected no α-synuclein Ser129 phosphorylation associated with neurofibrillary tangles in the hippocampus of a patient with Alzheimer′s disease (Waxman, E.A., et al. (2008). J. Neuropathol. Exp. Neurol. 67(5):402-416).
Immunofluorescence Analysis: A representative lot detected pathologic inclusions-associated α-synuclein Ser129 phosphorylation by fluorescent immunohistochemistry in paraffin-embedded cingulate cortex sections from a patient with LB variant of Alzheimer′s disease (LBVAD) and in the cerebellum sections from a patient with multiple systems atrophy (MSA) (Waxman, E.A., et al. (2008). J. Neuropathol. Exp. Neurol. 67(5):402-416).
Note: Incubating the transferred membrane with a combination of 4% paraformaldehyde and 0.01 ~ 0.1% glutaraldehyde is reported to produce an approximately 10-fold increase in the detection sensitivity of α-synuclein Ser129 phosphorylation by Western blotting. If not fixed, α-synuclein monomers can detach from the transferred membrane during incubation (Sasaki, A., et al. (2015). Sci. Rep. 5:14211).
Neuroscience
Neurodegenerative Diseases
Quality
Isotyping Analysis: The identity of this monoclonal antibody is confirmed by isotyping test to be IgG2aκ.
Target description
Physical form
Storage and Stability
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service