FC010
Human Fibronectin
from human plasma, liquid, 1 mg/mL, purified protein, suitable for cell culture
Synonym(s):
Fibronectin protein
About This Item
Recommended Products
product name
Human Plasma Fibronectin Purified Protein, from human plasma, liquid, 1 mg/mL (100 MG pack size is lyophilized), purified protein, suitable for cell culture
biological source
human
Quality Level
Assay
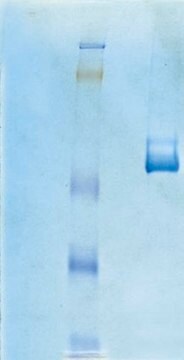
~95% (SDS-PAGE)
form
(liquid: 1MG, 5MG, 10MG pack size
lyophilized: 100 MG pack size)
mol wt
220 kDa
manufacturer/tradename
Chemicon®
concentration
1 mg/mL
technique(s)
cell culture | mammalian: suitable
surface coverage
2—10 μg/cm2
input
sample type mesenchymal stem cell(s)
sample type neural stem cell(s)
sample type: human embryonic stem cell(s)
sample type epithelial cells
sample type hematopoietic stem cell(s)
sample type induced pluripotent stem cell(s)
sample type pancreatic stem cell(s)
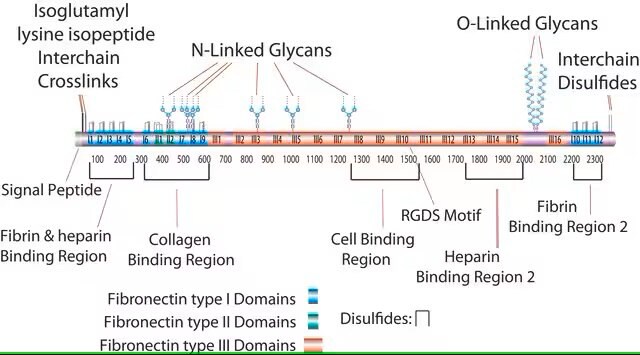
UniProt accession no.
Binding Specificity
Peptide Source: Collagen
Peptide Source: Laminin
shipped in
wet ice
storage temp.
2-8°C
Gene Information
human ... FN1(2335)
Related Categories
General description
Application
Biochem/physiol Actions
Physical form
Storage and Stability
Analysis Note
Other Notes
1.Determine the amount of HFN needed to coat culture vessels by multiplying the total surface area (cm2) by the desired concentration (μg/mL) of HFN. Recommended amount is 2-10 μg/cm2.
2.Wet the surface of each culture vessel to be coated with a minimum amount of sterile balanced salt solution (serum and protein free) required to cover the entire area.
3.Introduce the proper CO2 atmosphere, if required.
4.Add the calculated amount of HFN to each culture vessel.
5.Allow HFN to adsorb to the surface of the vessel for 5-20 minutes.
6.Remove residual balanced salt solution before proceeding with standard cell culture procedures
Product is filtered using a 0.1 micron filter during production. It is recommended to filter product again before use/after reconstitution.
Legal Information
Disclaimer
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The extracellular matrix (ECM) and its attachment factor components are discussed in this article in relation to their function in structural biology and their availability for in vitro applications.
The extracellular matrix (ECM) is secreted by cells and surrounds them in tissues.
Development of a novel serum-free and xeno-free human mesenchymal stem cell (MSC) osteocyte differentiation media.
Protocols
Dilute fibronectin for cell attachment, varying per cell type. Coating protocol, products, and FAQs provided.
This page covers the indirect co-culture of embryonic stem cells with embryonic fibroblasts.
Information about mesenchyme, specifically mesenchymal stem cell procotols. Step-by-step cell culture protocols for mesenchymal stem cell (MSC) isolation, expansion and differentiation.
This page covers the ECM coating protocols developed for four types of ECMs on Millicell®-CM inserts, Collagen Type 1, Fibronectin, Laminin, and Matrigel.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service