Select a Size
Select a Size
About This Item
Recommended Products
form
liquid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
refractive index
n20/D 1.599
density
1.017 g/mL at 25 °C
functional group
phosphine
SMILES string
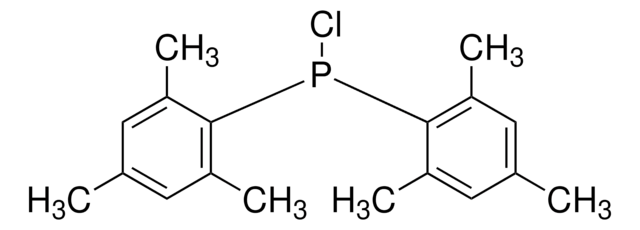
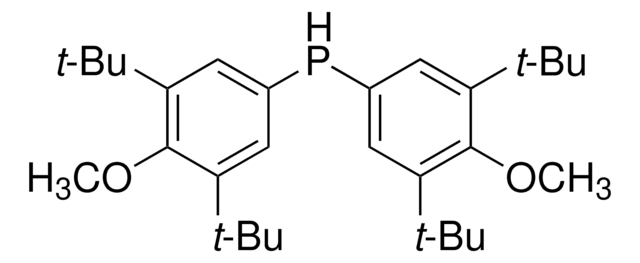
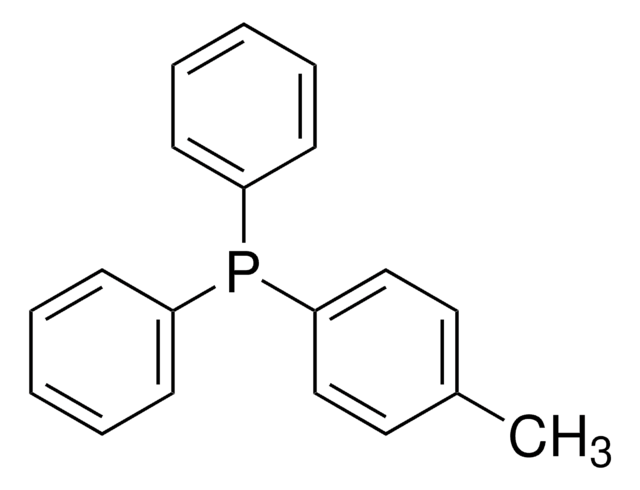
Cc1cc(C)cc(Pc2cc(C)cc(C)c2)c1
InChI
1S/C16H19P/c1-11-5-12(2)8-15(7-11)17-16-9-13(3)6-14(4)10-16/h5-10,17H,1-4H3
InChI key
GPFIUEZTNRNFGD-UHFFFAOYSA-N
1 of 4
This Item | 710385 | 392073 | 155039 |
|---|---|---|---|
| functional group phosphine | functional group phosphine | functional group phosphine | functional group phosphine |
| form liquid | form liquid | form liquid | form liquid |
| Quality Level 100 | Quality Level 100 | Quality Level 200 | Quality Level 100 |
| density 1.017 g/mL at 25 °C | density 1.141 g/cm3 at 25 °C | density 1.009 g/mL at 25 °C (lit.) | density - |
| refractive index n20/D 1.599 | refractive index n20/D 1.597 | refractive index n20/D 1.603 (lit.) | refractive index - |
Application
- Iron(II) chiral diimine diphosphine complexes as catalysts for the asymmetric transfer hydrogenation of ketones.[1]
- The complex of iridium with biphenol phosphite-phosphine bidentate ligand as an asymmetric catalyst for the hydrogenation of N-arylimines.[2]
- Chiral phosphines with excellent stereoselectivity by 1, 4 addition reaction with α,β-unsaturated aldehydes using a palladium catalyst.[3]
Legal Information
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service