All Photos(2)
About This Item
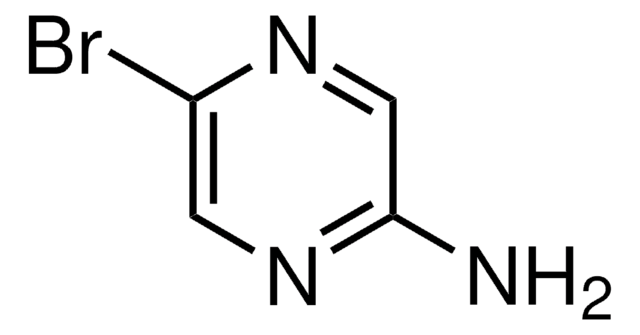
Empirical Formula (Hill Notation):
C5H5BrN2
CAS Number:
Molecular Weight:
173.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
88-91 °C (lit.)
SMILES string
Nc1cccc(Br)n1
InChI
1S/C5H5BrN2/c6-4-2-1-3-5(7)8-4/h1-3H,(H2,7,8)
InChI key
BKLJUYPLUWUEOQ-UHFFFAOYSA-N
Related Categories
General description
2-Amino- 6-bromopyridine has been synthesized from epichlorohydrin.
Application
2-Amino- 6-bromopyridine may be used in the synthesis of:
- 2-amino-6-diethylaminopyridine
- 2,6-di-(methylamino)-pyridine
- 3-(6-bromopyridin-2-yl)-2-(2,6-difluorophenyl)-1,3-thiazolidin-4-one
- N-(6-bromopyridin-2-yl)dodecylamide
- 2-bromo-6-iodopyridine
Employed in an efficient one-pot synthesis of 7-azaindoles.
Used in the synthesis of anti-HIV agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
II. Derivatives of 2, 6-Diaminopyridine1.
Bernstein J, et al.
Journal of the American Chemical Society, 69(5), 1151-1115 (1947)
Synthesis of ?Acetylene-Expanded? Tridentate Ligands
Holmes BT, et al.
Molecules (Basel), 7(5), 447-455 (2002)
Masato Ikeda et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(2), 662-668 (2004-11-27)
The bow-shaped molecule 1 bearing a self-complementary DAAD-ADDA (D=donor A=acceptor) hydrogen-bonding array generates, in hydrocarbon solvents, highly ordered supramolecular sheet aggregates that subsequently give rise to gels by formation of an entangled network. The process of hierarchical self-assembly of compound
Maria Letizia Barreca et al.
Farmaco (Societa chimica italiana : 1989), 58(3), 259-263 (2003-03-07)
This paper reports our work in the field of nonnucleoside RT inhibitors (NNRTIs). On the basis of extensive studies on 1H,3H-thiazolo[3,4-a]benzimidazole derivatives (TBZs) followed by structure-activity relationship (SAR) considerations and molecular modeling, the design and synthesis of a series of
Microwave-assisted synthesis of benzimidazole and thiazolidinone derivatives as HIV-1 RT inhibitors.
Rao A, et al.
ARKIVOC (Gainesville, FL, United States), 147, 155-155 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service