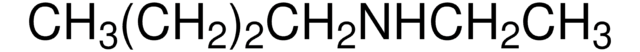369527
N,N-Dimethylbutylamine
99%
Synonym(s):
N-Butyldimethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3N(CH3)2
CAS Number:
Molecular Weight:
101.19
Beilstein:
1731308
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
99%
refractive index
n20/D 1.398 (lit.)
bp
93.3 °C/750 mmHg (lit.)
density
0.721 g/mL at 25 °C (lit.)
SMILES string
CCCCN(C)C
InChI
1S/C6H15N/c1-4-5-6-7(2)3/h4-6H2,1-3H3
InChI key
DJEQZVQFEPKLOY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N,N-Dimethylbutylamine is a tertiaryalkylamine. X-ray photoelectron spectra (XPS) of Si(001) surfaces exposed to N,N-dimethylbutylamine at 300K has been reported.
Application
N,N-Dimethylbutylamine is suitable for use in the fabrication of polystyrene-based nano-LC monolithic columns for the separation of protein molecules. It may be used as ion-pairing reagent in a study involving comparison of performance of six ion-pairing reagents as mobile phase modifiers for oligonucleotide LC/MS.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
23.0 °F - closed cup
Flash Point(C)
-5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Leta Deressa Tolesa et al.
International journal of biological macromolecules, 130, 818-826 (2019-03-07)
Ammonium-based ionic liquids (ILs): diisopropylethylammonium acetate ([DIPEA][Ac]), diisopropylethylammonium propanoate ([DIPEA][P]) and dimethylbutylammonium acetate ([DMBA][Ac]) are used for the extraction of chitin from shrimp shells. The extracted chitins were characterized by FT-IR, TGA, XRD, SEM and 1H NMR. The yield of
Joshua E S J Reid et al.
Physical chemistry chemical physics : PCCP, 19(41), 28133-28138 (2017-10-13)
The ionic nature of a functionalized protic ionic liquid cannot be rationalized simply through the differences in aqueous proton dissociation constants between the acid precursor and the conjugate acid of the base precursor. The extent of proton transfer, i.e. the
Łukasz Nuckowski et al.
The Analyst, 144(15), 4622-4632 (2019-06-28)
Our research focused on applying microextraction by packed sorbent to extracting antisense oligonucleotides from serum samples. The tested sorbents included poly(styrene-co-divinylbenzene), octyl, octadecyl, and unmodified silica gel. As nonpolar sorbents were used for highly-polar molecules, this required ion-pair mode. Comprehensive
Sylwia Studzińska et al.
Analytical and bioanalytical chemistry, 408(6), 1585-1595 (2016-01-14)
Ultra high performance liquid chromatography hyphenated with quadrupole time-of-flight mass spectrometry was used to determine the products of the in vitro metabolism of phosphorothioate oligonucleotides. These compounds may be used during antisense therapy as synthetic fragments of genes. For this
Anna Kilanowska et al.
Analytical and bioanalytical chemistry, 412(27), 7453-7467 (2020-08-29)
The aim of the present investigation was the analysis and identification of antisense oligonucleotide metabolism products after incubation with human liver microsomes regarding four different oligonucleotide modifications. Separation and detection methods based on the use of liquid chromatography coupled with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service