336572
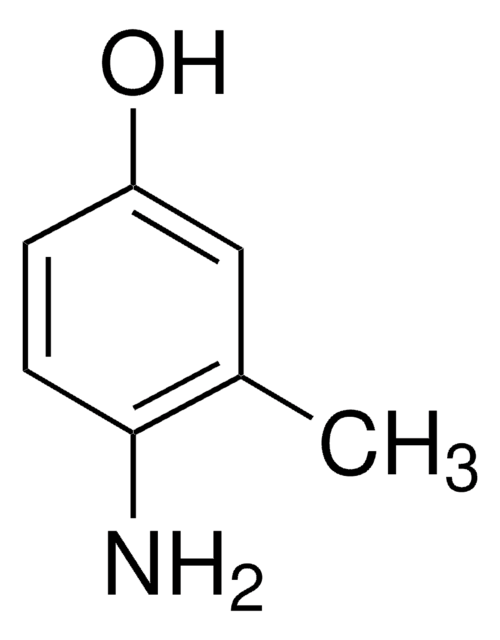
2-Amino-3-methylphenol
96%
Synonym(s):
2-Amino-m-cresol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H3(CH3)OH
CAS Number:
Molecular Weight:
123.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
149-152 °C (lit.)
SMILES string
Cc1cccc(O)c1N
InChI
1S/C7H9NO/c1-5-3-2-4-6(9)7(5)8/h2-4,9H,8H2,1H3
InChI key
FEDLEBCVFZMHBP-UHFFFAOYSA-N
General description
2-Amino-3-methylphenol is an aminophenol formed by incubating isopropyl-β-D-thiogalactopyranoside-induced E. coli JS996 strain cells with various nitroaromatic compounds.
Application
2-Amino-3-methylphenol was used in the preparation of Schiff base ligands.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Construction of Escherichia coli strains for conversion of nitroacetophenones to ortho-aminophenols.
Venkateswarlu Kadiyala et al.
Applied and environmental microbiology, 69(11), 6520-6526 (2003-11-07)
The predominant bacterial pathway for nitrobenzene (NB) degradation uses an NB nitroreductase and hydroxylaminobenzene (HAB) mutase to form the ring-fission substrate ortho-aminophenol. We tested the hypothesis that constructed strains might accumulate the aminophenols from nitroacetophenones and other nitroaromatic compounds. We
Synthesis and reactivity of novel cyclometallated complexes derived from [C, N, O] terdentate ligands. Crystal structure of [Pd {2, 3, 4-(MeO) 3 C 6 HC (H)[double bond, length half m-dash] N [2-(O) C 6 H 4]}(PPh 3)].
Fernandez A, et al.
New. J. Chem., 26(4), 398-404 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service