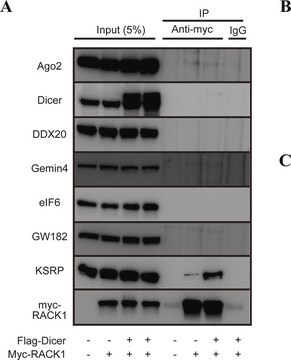
All Photos(1)
About This Item
Linear Formula:
CH3CHF2
CAS Number:
Molecular Weight:
66.05
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.28 (vs air)
Assay
≥98%
bp
−25 °C (lit.)
mp
−117 °C (lit.)
SMILES string
CC(F)F
InChI
1S/C2H4F2/c1-2(3)4/h2H,1H3
InChI key
NPNPZTNLOVBDOC-UHFFFAOYSA-N
Packaging
Supplied in a Sure/Pac™ cylinder and has a brass needle valve with a male 1/4" NPTF outlet thread installed. Before using the cylinder, ensure that the valve is closed, then remove the galvanized steel hex cap that seals the outlet valve.
Compatible with the following:
Compatible with the following:
Legal Information
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
Sure/Pac is a trademark of Sigma-Aldrich Co. LLC
also commonly purchased with this product
Product No.
Description
Pricing
hose barb
Product No.
Description
Pricing
recommended
Product No.
Description
Pricing
regulator
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Joseph Avella et al.
Journal of analytical toxicology, 34(7), 381-388 (2010-09-09)
1,1-Difluoroethane (DFE) is a halogenated hydrocarbon used as a propellant in products designed for dusting electronic equipment and air brush painting. When abused, inhaled DFE produces intoxication and loss of muscular coordination. To investigate DFE toxicokinetics, groups (n = 3)
K S Sidhu et al.
Veterinary and human toxicology, 31(1), 63-64 (1989-02-01)
A veterinary technician while opening a package was accidentally exposed to a commercial canned product formulation containing insecticides and solvents. The patient was twice briefly treated and released as an outpatient from 2 different hospitals on the first and second
E Gridelet et al.
The journal of physical chemistry. A, 109(37), 8225-8235 (2006-07-13)
The kinetic energy release distributions (KERDs) for the fluorine atom loss from the 1,1-difluoroethene cation have been recorded with two spectrometers in two different energy ranges. A first experiment uses dissociative photoionization with the He(I) and Ne(I) resonance lines, providing
Timothy Hahn et al.
Journal of analytical toxicology, 30(8), 638-642 (2006-11-30)
A 24-year-old female driver with a history of substance abuse was pronounced dead following a single car motor vehicle accident. A surviving front seat passenger witnessed the decedent inhaling "Dust Off" cleaner just prior to losing control of the vehicle.
Kevin N Foster et al.
The Journal of burn care & rehabilitation, 24(4), 234-237 (2003-09-23)
A young female suffered burns to her hand after the ignition of gas from a compressed air duster. After debridement and dressing, the patient continued to have pain out of proportion to injury that was refractory to intravenous morphine. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service