All Photos(1)
About This Item
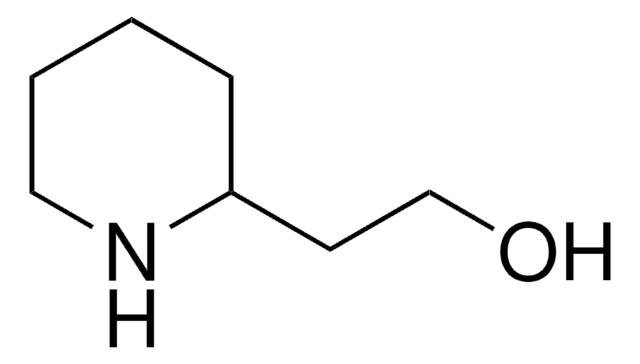
Empirical Formula (Hill Notation):
C11H9N
CAS Number:
Molecular Weight:
155.20
Beilstein:
110400
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.616 (lit.)
bp
269-270 °C/749 mmHg (lit.)
density
1.082 g/mL at 25 °C (lit.)
SMILES string
c1ccc(cc1)-c2cccnc2
InChI
1S/C11H9N/c1-2-5-10(6-3-1)11-7-4-8-12-9-11/h1-9H
InChI key
HJKGBRPNSJADMB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Phenylpyridine forms complexes with gold(III), palladium(II) and platinum(II) chloride and their 1H, 13C and 15N nuclear magnetic resonance studies have been reported. Interactions of 3-phenylpyridine with copper surface has been investigated by Surface-enhanced Raman scattering (SERS) spectroscopy and cyclic voltammetry. It is a useful synthetic intermediate.
Application
3-Phenylpyridine was employed as axial ligand to investigate the rate of olefin oxygenation catalyzed by synthetic metalloporphyrins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Surface-enhanced Raman spectroscopy studies of phenylpyridines interacting with a copper electrode surface.
Zawada K and Bukowska J.
Surface Science, 507, 34-39 (2002)
T L Perry et al.
Journal of the neurological sciences, 85(3), 309-317 (1988-07-01)
Idiopathic Parkinson's disease (PD) is likely to be caused by one or more unidentified neurotoxins, present in the environment or formed endogenously, which progressively damage dopaminergic nigrostriatal neurons. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is an experimental neurotoxin which produces biochemical and neuropathological changes
Marc P Bonaca et al.
Future cardiology, 5(5), 435-442 (2009-09-01)
SCH 530348, a synthetic tricyclic 3-phenylpyridine, is an orally active fourth generation himbacine-based antagonist of the protease-activated receptor (PAR)-1, the primary receptor for thrombin on platelets in humans. SCH 530348 is the first in a new class of compounds that
Sanjeev Kumar et al.
Journal of medicinal chemistry, 51(16), 4968-4977 (2008-07-31)
Indoleamine 2,3-dioxygenase (IDO) is emerging as an important new therapeutic target for the treatment of cancer, chronic viral infections, and other diseases characterized by pathological immune suppression. With the goal of developing more potent IDO inhibitors, a systematic study of
Leszek Pazderski et al.
Magnetic resonance in chemistry : MRC, 47(8), 658-665 (2009-05-28)
1H, 13C and 15N nuclear magnetic resonance studies of gold(III), palladium(II) and platinum(II) chloride complexes with phenylpyridines (PPY: 4-phenylpyridine, 4ppy; 3-phenylpyridine, 3ppy; and 2-phenylpyridine, 2ppy) having the general formulae [Au(PPY)Cl3], trans-/cis-[Pd(PPY)2Cl2] and trans-/cis-[Pt(PPY)2Cl2] were performed and the respective chemical shifts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service