194654
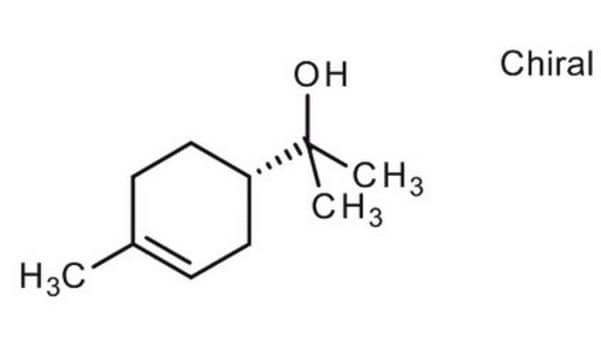
Chrysanthemyl alcohol, mixture of cis and trans
98%
Synonym(s):
2,2-Dimethyl-3-(2-methylpropenyl)cyclopropanemethanol, Chrysanthemol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C3H2[CH=C(CH3)2]CH2OH
CAS Number:
Molecular Weight:
154.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
refractive index
n20/D 1.475 (lit.)
bp
66-69 °C/0.07 mmHg (lit.)
density
0.888 g/mL at 25 °C (lit.)
SMILES string
C\C(C)=C\C1C(CO)C1(C)C
InChI
1S/C10H18O/c1-7(2)5-8-9(6-11)10(8,3)4/h5,8-9,11H,6H2,1-4H3
InChI key
HIPIENNKVJCMAP-UHFFFAOYSA-N
General description
Oxidation of chrysanthemyl alcohol in the presence of [RuO4]2- yields chrysantheniic acid.
Application
Chrysanthemyl alcohol was used in the determination of position of double bonds in various terepenes and branched chain compounds.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A B Attygalle et al.
Analytical chemistry, 65(18), 2528-2533 (1993-09-15)
The electron-impact mass spectra of alpha, beta-bis(methylthio) derivatives of certain terpenes and other compounds with branched alkenyl groups contain diagnostic peaks that can be used for locating the position of the double bond in the parent compound. In contrast to
Oxo complexes of ruthenium (VI) and (VII) as organic oxidants.
Green G, et al.
Journal of the Chemical Society. Perkin Transactions 1, 681-686 (1984)
L Y Mou et al.
Journal of Asian natural products research, 3(2), 103-116 (2001-06-16)
Chrysanthemol (1), a trans-eudesmane type sesquiterpene from Chrysanthemum indicum L., possesses certain anti-inflammatory activity. Its total synthesis was approached from two alternative routes and finally accomplished in ten steps from R-(+)-carvone via alpha-eudesmol (10) as the key intermediate. The overall
[Studies on the chemical constituents of Chrysanthemum indicum L].
D Q Yu et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 22(11), 837-840 (1987-11-01)
Gas chromatographic determination of racemic cis- and trans-chrysanthemols and their potential aldehyde and carboxylic acid microbial metabolites.
M Miski et al.
Journal of chromatography, 437(2), 436-441 (1988-03-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service