All Photos(2)
About This Item
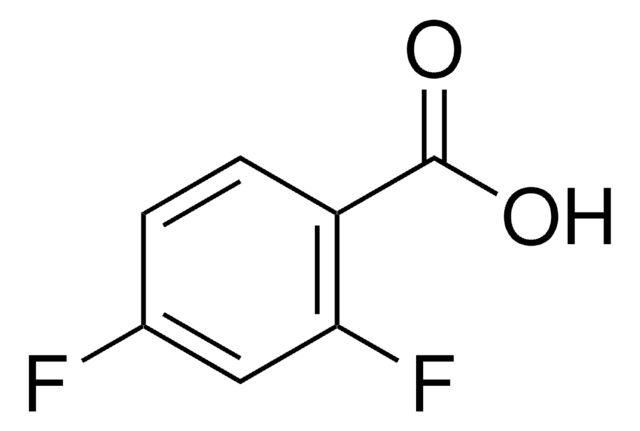
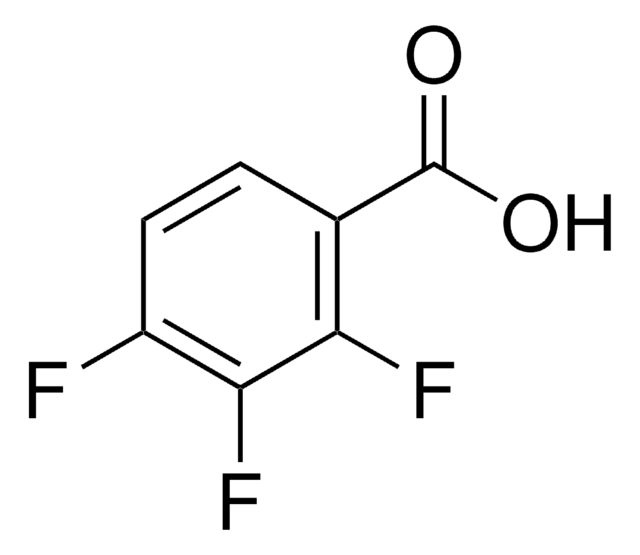
Linear Formula:
F2C6H3CO2H
CAS Number:
Molecular Weight:
158.10
Beilstein:
973774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
157-161 °C (lit.)
SMILES string
OC(=O)c1c(F)cccc1F
InChI
1S/C7H4F2O2/c8-4-2-1-3-5(9)6(4)7(10)11/h1-3H,(H,10,11)
InChI key
ONOTYLMNTZNAQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Difluorobenzoic acid is the major degradation product of diflubenzuron.
Application
2,6-Difluorobenzoic acid has been used in the synthesis of 2,6-difluoro-N-(3-methoxy-1H-pyrazolo[3,4-b]pyridine-5-yl)-3-(propylsulfonamidio)benzamide and methyl 2,6-difluorobenzoate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Gattavecchia et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 16(2), 159-166 (1981-01-01)
Diflubenzuron (I) and its major degradation products 4-chlorophenyl urea (II), 2,6-difluorobenzoic acid (III) and 4-chloroaniline (IV) were tested for their activity on Euglena gracilis Z. The inhibition on the growth and on the incorporation of glycine-U-14C in the protein of
A Karipides et al.
Acta crystallographica. Section C, Crystal structure communications, 48 ( Pt 6), 1015-1018 (1992-06-15)
Ca2+.2C7H3O2F2-.2H2O, M(r) = 390.3, monoclinic, C2/c, a = 17.584 (4), b = 10.771 (3), c = 7.887 (2) A, beta = 91.28 (2) degrees, V = 1493 A3, Z = 4, Dm = 1.75, Dx = 1.74 g cm-3, lambda(Mo
Li Chen et al.
Journal of agricultural and food chemistry, 53(1), 38-41 (2005-01-06)
Two series of benzoylphenylurea derivatives were synthesized as candidate propesticides by a nucleophilic addition reaction between 2,6-difluronbenzoyl isocyanate and N-substitutedaniline. The new compounds were identified by 1H NMR spectroscopy, electron ionization-mass spectrometry, and elemental analyses. The bioactivities of the new
Min Wang et al.
Bioorganic & medicinal chemistry letters, 23(4), 1017-1021 (2013-01-09)
The authentic standard 2,6-difluoro-N-(3-methoxy-1H-pyrazolo[3,4-b]pyridine-5-yl)-3-(propylsulfonamidio)benzamide was synthesized from 2,6-difluorobenzoic acid and 3-amino-5-hydroxypyrazole in 9 steps with 1% overall chemical yield. Direct desmethylation of the reference standard with TMSCl/NaI gave the precursor 2,6-difluoro-N-(3-hydroxy-1H-pyrazolo[3,4-b]pyridine-5-yl)-3-(propylsulfonamidio)benzamide for radiolabeling in 70% yield. The target tracer 2,6-difluoro-N-(3-[(11)C]methoxy-1H-pyrazolo[3,4-b]pyridine-5-yl)-3-(propylsulfonamidio)benzamide
J Koerts et al.
Xenobiotica; the fate of foreign compounds in biological systems, 27(8), 801-817 (1997-08-01)
1. The metabolic fate of the insecticide teflubenzuron, orally dosed to the male Wistar rat, was investigated. Particular attention was paid to the metabolic fate of the benzoyl and aniline moiety after hydrolysis of the urea bridge. 2. The 0-48-h
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service