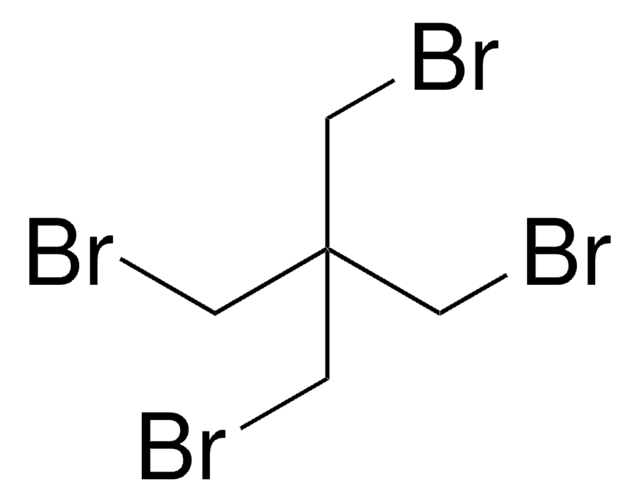
185140
3,3′-Dichloropivalic acid
≥97%
Synonym(s):
2,2-Bis(chloromethyl)propionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(CH2Cl)2CO2H
CAS Number:
Molecular Weight:
171.02
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
form
solid
mp
64-66 °C (lit.)
SMILES string
CC(CCl)(CCl)C(O)=O
InChI
1S/C5H8Cl2O2/c1-5(2-6,3-7)4(8)9/h2-3H2,1H3,(H,8,9)
InChI key
DDSPBKFTRPWDLI-UHFFFAOYSA-N
Related Categories
General description
3,3′-Dichloropivalic acid reacts with ethane-1,2-diamine to yield isomeric tetra-amine derivatives, tetra amino carboxylic acid and carboxamidotriamino alcohol.
Application
3,3′-Dichloropivalic acid was used in the synthesis of 3,3′-dichloropivaloyl chloride.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Paul V Bernhardt et al.
Inorganic chemistry, 43(5), 1681-1688 (2004-03-03)
Reaction between ethane-1,2-diamine and 3,3'-dichloropivalic acid results in different, isomeric tetra-amine derivatives, one a tetraamino carboxylic acid and the other a carboxamidotriamino alcohol, depending upon reaction conditions. Intended conversion of the Cu(II) complex of the former to a cyclam-like macrocycle
A novel rearrangement reaction conversion of 3-(chloromethyl) azetidin-2-ones to azetidine-3-carboxylic acid esters.
Bartholomew D and Stocks MJ.
Tetrahedron Letters, 32(36), 4795-4798 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service