178098
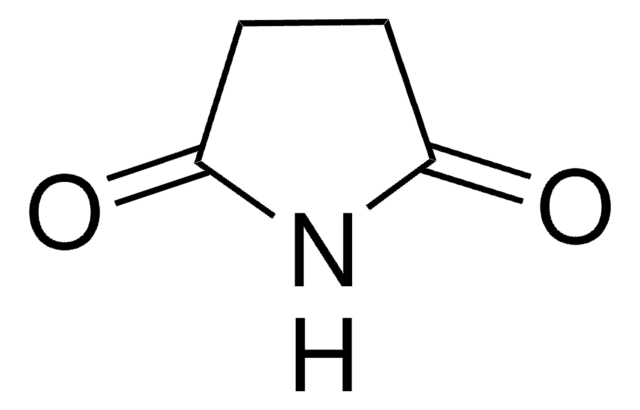
Glutarimide
98%
Synonym(s):
2,6-Piperidinedione, NSC 58190
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
155-157 °C (lit.)
SMILES string
O=C1CCCC(=O)N1
InChI
1S/C5H7NO2/c7-4-2-1-3-5(8)6-4/h1-3H2,(H,6,7,8)
InChI key
KNCYXPMJDCCGSJ-UHFFFAOYSA-N
General description
A glutarimide antibiotic, 9-methylstreptimidone, shows antiviral, antitumor and antifungal activities.
Application
Reactant for:
Thionations
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture
Synthesis of β-adrenoceptor ligands
Enantioselective synthesis of securinega alkaloids
Intramolecular amidocyclopropanation reactions
Synthesis of alpha-fluoro-alpha amino amides
Thionations
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture
Synthesis of β-adrenoceptor ligands
Enantioselective synthesis of securinega alkaloids
Intramolecular amidocyclopropanation reactions
Synthesis of alpha-fluoro-alpha amino amides
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matthew Hoffmann et al.
Cancer chemotherapy and pharmacology, 71(2), 489-501 (2012-12-04)
To investigate the pharmacokinetics and disposition of [(14)C]pomalidomide following a single oral dose to healthy male subjects. Eight subjects were administered a single 2 mg oral suspension of [(14)C]pomalidomide. Blood (plasma), urine and feces were collected. Mass balance of radioactivity
Cai-Yun Geng et al.
Journal of computational chemistry, 29(5), 686-693 (2007-09-13)
The reaction mechanism of the Rh-catalyzed [4 + 2] annulation of 4-alkynals with isocyanates is unraveled using density functional calculations. The reaction mechanisms of the model system and the real substituted system have been investigated and the results are compared.
Chuanjin Tian et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(45), 14305-14313 (2012-10-16)
The significance of the molecular chirality of drugs has been widely recognized due to the thalidomide tragedy. Most of the new drugs reaching the market today are single enantiomers, rather than racemic mixtures. However, many optically pure drugs, including thalidomide
Pei-Qiang Huang et al.
Organic letters, 8(7), 1435-1438 (2006-03-28)
[reaction: see text] Using 5b as a common intermediate, the first asymmetric synthesis of (-)-epiquinamide (4) and a formal asymmetric synthesis of (-)-homopumiliotoxin 223G (2) is described. A key feature of our approach is the flexible introduction of a functionalized
Deevi Basavaiah et al.
Organic & biomolecular chemistry, 6(6), 1034-1039 (2008-03-11)
A simple and convenient synthesis of di(E)-arylidene-tetralone-spiro-glutarimides from Baylis-Hillman acetates via an interesting biscyclization strategy involving facile C-C and C-N bond formation is described. Also, one-pot multistep transformation of the Baylis-Hillman acetates into di(E)-arylidene-spiro-bisglutarimides is presented.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service