All Photos(1)
About This Item
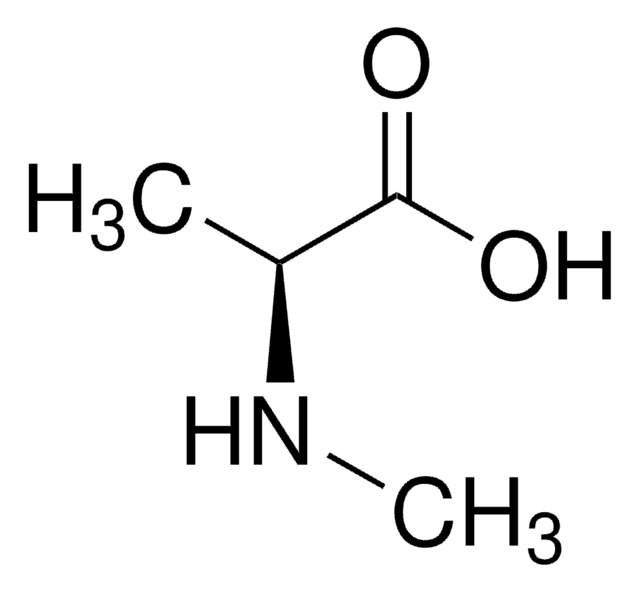
Empirical Formula (Hill Notation):
C4H9NO2
CAS Number:
Molecular Weight:
103.12
MDL number:
UNSPSC Code:
12352200
eCl@ss:
32160406
PubChem Substance ID:
Recommended Products
Assay
≥98% (TLC)
form
powder
color
white
SMILES string
CNC(C)C(O)=O
InChI
1S/C4H9NO2/c1-3(5-2)4(6)7/h3,5H,1-2H3,(H,6,7)
InChI key
GDFAOVXKHJXLEI-UHFFFAOYSA-N
Biochem/physiol Actions
N-Methyl-DL-alanine may be used in studies of amino acid transport mechanisms such as trans-stimulation. N-Methylalanine may be used in the construction of peptide and protein analogues for use in drug or antibiotic design and development.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bang-zhi Zhang et al.
Peptides, 31(4), 568-573 (2010-01-05)
The actinomycin D (AMD) analogs in which the D-valine residues (the second amino acid residue in the cyclic depsipeptide of AMD) and the N-methyl-L-valine residues (the fifth amino acid residue in the cyclic depsipeptide of AMD) were replaced with D-Phe
G E Mann et al.
The Journal of physiology, 416, 485-502 (1989-09-01)
1. Epithelial uptake and efflux of the non-metabolized system A analogue 2-methylaminoisobutyric acid (MeAIB) and L-serine were studied in the isolated perfused rat pancreas using a dual tracer loading and wash-out technique. Uptakes of 2-[14C]MeAIB and L-[3H]serine were measured relative
Renata Perlikowska et al.
Basic & clinical pharmacology & toxicology, 106(2), 106-113 (2009-10-31)
A series of endomorphin-1 (EM-1) and endomorphin-2 (EM-2) analogues, containing non-cyclic amino acids (Ala, D-Ala, beta-Ala, NMeAla, D-NMeAla or Sar) instead of Pro in position 2 was synthesized, where NMeAla = N-methylalanine and Sar = N-methylglycine, sarcosine. The opioid activity
D Gazis et al.
International journal of peptide and protein research, 23(1), 78-83 (1984-01-01)
Substituting sarcosine or N-methylalanine for proline in the inhibitory vasopressin analogs dPAVP and d(CH2)5AVP had the following effects: 1) milk ejection and antidiuretic activities were severely depressed, 2) pressor antagonism was maintained but weakened somewhat, and 3) antagonism in the
Z Grzonka et al.
International journal of peptide and protein research, 25(4), 375-381 (1985-04-01)
The 600 MHz proton n.m.r. spectra of (sarcosyl7)-oxytocin and (N-methylalanyl7) oxytocin in 2H2O solution have been recorded and completely assigned. In each case the spectrum indicates the presence of two slowly interconverting conformers, which are the cis-trans isomers about the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service