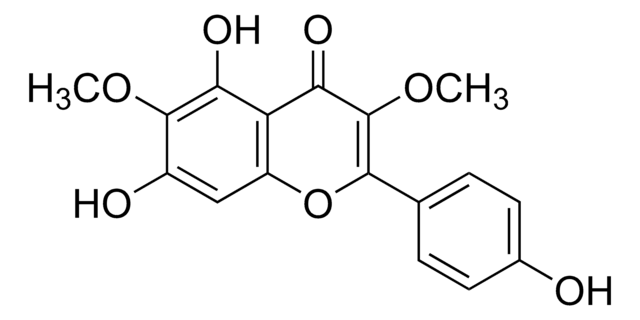
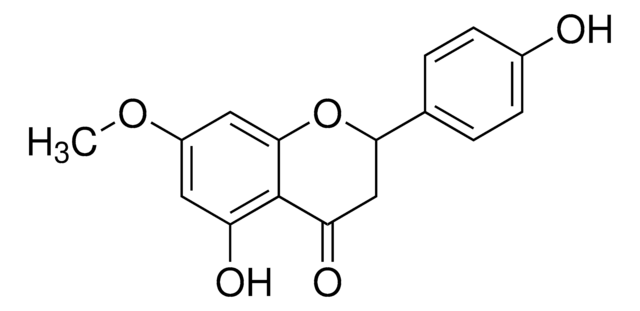
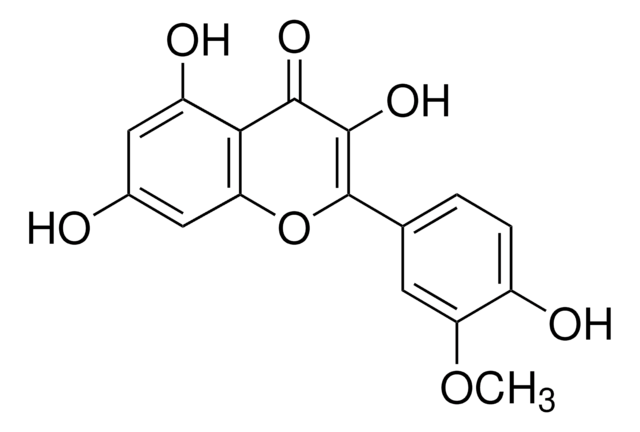
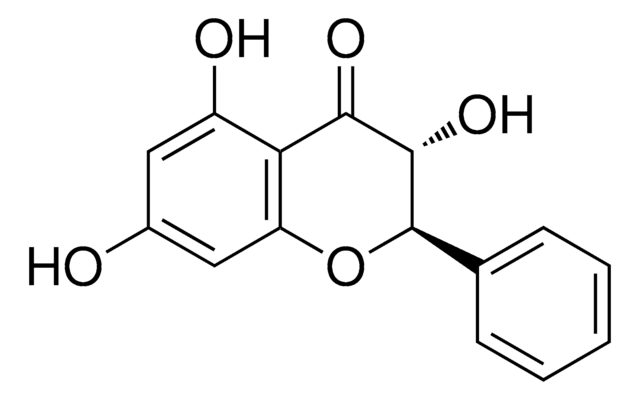
Isorhamnetin shares structural similarities with 3-O-methylquercetin. They are structural isomers, sharing similar chemical compositions but differing in the arrangement of atoms. Both compounds belong to the flavonoid family and have various dietary sources.
For Item 90081, the structure and properties (SMILES string, InChI, InChI key) are of 3-O-methylquercetin per PubChem: https://pubchem.ncbi.nlm.nih.gov/compound/5280681.