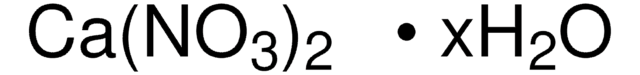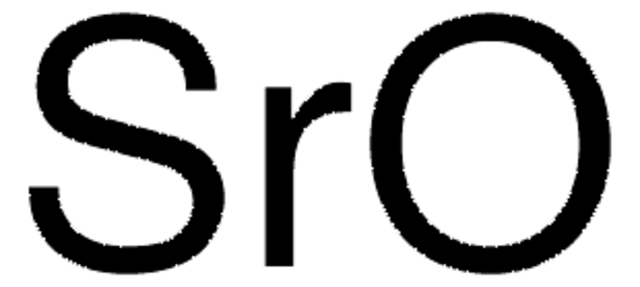About This Item
Recommended Products
grade
ACS reagent
Quality Level
Assay
≥99.0%
form
crystals or chunks
impurities
≤0.01% insolubles
loss
≤0.1% loss on drying
pH
5.0-7.0 (25 °C, 5%)
mp
570 °C (lit.)
anion traces
chloride (Cl-): ≤0.002%
sulfate (SO42-): ≤0.005%
cation traces
Ba: ≤0.05%
Ca: ≤0.05%
Fe: ≤5 ppm
Mg: ≤0.10%
Na: ≤0.10%
heavy metals: ≤5 ppm (by ICP-OES)
SMILES string
[Sr++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/2NO3.Sr/c2*2-1(3)4;/q2*-1;+2
InChI key
DHEQXMRUPNDRPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Synthesis and Antimicrobial Analysis of High Surface Area Strontium-Substituted Calcium Phosphate Nanostructures for Bone Regeneration: The study explores the potential of strontium nitrate in bone regeneration through the synthesis of high surface area strontium-substituted calcium phosphate nanostructures with antimicrobial properties (Anwar et al., 2023).
- Electrochemically Assisted Deposition of Strontium Modified Magnesium Phosphate on Titanium Surfaces: The article describes the use of strontium nitrate in creating coatings on titanium surfaces for improved biomedical applications, enhancing material properties through electrochemical deposition techniques (Meininger et al., 2016).
- Production of Y-86 and Other Radiometals for Research Purposes Using a Solution Target System: This study involves the use of strontium nitrate in the production of radiometals, including Y-86, for various research and diagnostic purposes, employing a solution target system for effective synthesis (Oehlke et al., 2015).
- Template-based Fabrication of SrTiO3 and BaTiO3 Nanotubes: The research discusses the utility of strontium nitrate in the template-based fabrication of nanotubes, specifically SrTiO3 and BaTiO3, which are critical in enhancing material properties for various industrial and technological applications (Chen et al., 2009).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Ox. Sol. 1
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service